ISSUE1385
The FDA has approved a new tablet formulation of immediate-release (IR) oxycodone (Oxecta – King) for management of acute and chronic moderate to severe pain.
Oxecta uses a tamper-resistant technology designed to deter oxycodone abuse by injection or nasal snorting. Dissolving the crushed tablet in water or alcohol converts it into a viscous gel mixture, making it difficult to inject. Crushing the tablet and inhaling it through the nose causes burning and irritation. Whether the new formulation will actually prevent abuse of the drug has not been established. Oxecta is classified as a Schedule II controlled substance.1
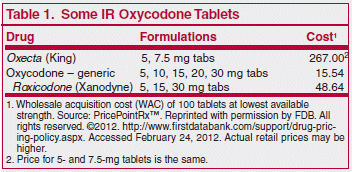
In patients who have not been receiving opioid analgesics, the recommended starting dosage of Oxecta is 5 to 15 mg every 4 to 6 hours as needed for pain. Oxycodone has no analgesic ceiling, but dosage is usually limited by its adverse effects. Use of similar abuse-resistant tablets has been associated with reports of difficulty swallowing the tablets.2 Oxecta tablets should be swallowed whole and taken with enough water to ensure complete swallowing.
