ISSUE1210
Picaridin (KBR 3023), which has been used as an insect repellent for years in Europe and Australia (Autan Repel, and others), is now available in the US in a 7% solution as Cutter Advanced (Spectrum Brands). The US Centers for Disease Control and Prevention (CDC) is recommending it as an alternative to DEET.
DEET — DEET is available in the US in many formulations with concentrations of 5%-40% and 100%; higher concentrations offer complete protection for a longer period of time, but the duration of effectiveness reaches a plateau at a 50% concentration. A long-acting DEET formulation, originally developed for the US Armed Forces (US Army Extended Duration Topical Insect and Arthropod Repellent, EDTIAR) is available in the US as Ultrathon (3M). Ultrathon contains 25% (in aerosol) or 33% (in cream) DEET in a polymer formulation, which prevents loss from the skin surface through absorption and evaporation. Studies have shown that it provides complete protection against mosquitoes for 6-12 hours.
Safety and Acceptability – Despite earlier concerns, toxic and allergic reactions to DEET have been uncommon, and serious adverse effects are rare.1 Some patients dislike its odor and find it irritating or uncomfortably oily or sticky on the skin. DEET can damage clothes made from synthetic fibers such as spandex or rayon and can also damage leather and plastics on eyeglass frames and watch crystals.
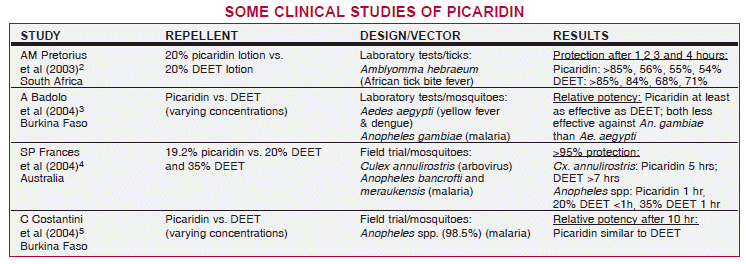
PICARIDIN — Laboratory and field studies documenting the efficacy of picaridin are summarized in the table on page 47. No published data are available on the efficacy of the 7% solution now available in the US. Insect repellents are registered by the Environmental Protection Agency (EPA); they do not require FDA approval.
Safety and Acceptability – In Australia and in Europe, no serious toxicity has been reported with picaridin. It has shown no evidence of dermal, organ or reproductive toxicity or carcinogenicity in animals, except for some hepatic toxicity in rats at extremely high doses.6,7 Unlike DEET, it is odorless, does not feel greasy or sticky, is less likely to irritate the skin, and does not damage plastics or fabrics.
DOSAGE AND COST—The manufacturer recommends spraying picaridin on the skin every 3 to 4 hours. Cutter Advanced (with 7% picaridin) is the only formulation available commercially in the US; a 6-ounce pump-spray bottle can be purchased over the counter for about $4.
CONCLUSION — The 7% picaridin formulation currently sold in the US might be as effective in repelling mosquitoes as low concentrations of DEET, but no data are available. Higher strength products sold in Europe (with 20% picaridin) protect against mosquitoes for up to 8 hours and against ticks for a shorter period of time. If higher concentrations become available in the US, picaridin could replace DEET due to its superior tolerability, but its long-term safety is less well established.
