The Medical Letter on Drugs and Therapeutics
FROM
ISSUE1519
ISSUE1519
Med Lett Drugs Ther. 2017 Apr 24;59(1519):70
Disclosures
Objective(s)
The 2017 adult immunization schedule approved by the CDC's Advisory Committee on Immunization Practices (ACIP) includes some new or revised recommendations.1 The complete schedule is available on the CDC's website (www.cdc.gov/vaccines/schedules). New recommendations for use of influenza vaccine during the 2016-2017 season were included in a previous issue of The Medical Letter.2 Updated recommendations for other vaccines are summarized below. Recommendations for routine use of vaccines in adults were reviewed in an earlier issue.3
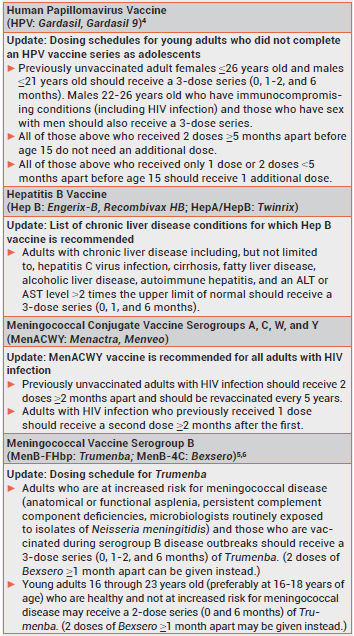
- DK Kim et al. Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older – United States, 2017. MMWR Morb Mortal Wkly Rep 2017; 66:136.
- Influenza vaccine for 2016-2017. Med Lett Drugs Ther 2016; 58:127.
- Adult immunization. Treat Guidel Med Lett 2014; 12:39.
- Gardasil 9 – a broader HPV vaccine. Med Lett Drugs Ther 2015; 57:47.
- Trumenba: a serogroup B meningococcal vaccine. Med Lett Drugs Ther 2015; 57:5.
- Bexsero – a second serogroup B meningococcal vaccine. Med Lett Drugs Ther 2015; 57:158.
© The Medical Letter, Inc. All Rights Reserved.
The Medical Letter, Inc. does not warrant that all the material in this publication is accurate and
complete in every respect. The Medical Letter, Inc. and its editors shall not be held responsible for any
damage resulting from any error, inaccuracy, or omission.
