ISSUE1623
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of vibegron (Gemtesa) for treatment of overactive bladder.
- FDA-approved for treatment of overactive bladder in adults with symptoms of urge urinary incontinence, urgency, and urinary frequency.
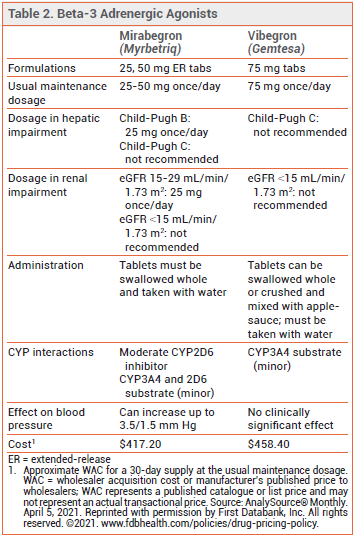
- Second beta-3 agonist to be approved for treatment of overactive bladder; mirabegron (Myrbetriq) was the first.
- Decreased mean daily micturitions compared to placebo and appeared to be similar in efficacy to the anticholinergic drug tolterodine in a double-blind clinical trial.
- Unlike mirabegron, vibegron has not been associated with blood pressure elevations.
- Taken once daily with a glass of water; tablets can be swallowed whole or crushed and mixed with applesauce.
Outline
Tables |
The FDA has approved the selective beta-3 adrenergic agonist vibegron (Gemtesa – Urovant Sciences) for treatment of overactive bladder in adults with symptoms of urge urinary incontinence, urgency, and urinary frequency. It is the second beta-3 agonist to be approved in the US; mirabegron (Myrbetriq) was the first.1

STANDARD TREATMENT — Overactive bladder (OAB) causes urgency, frequency, nocturia, and incontinence. It occurs most commonly in older women. Management with behavioral modification, including bladder training, urge suppression, pelvic floor muscle exercises, and avoidance of dietary irritants such as alcohol and caffeine, should be tried first.
Anticholinergic drugs, such as tolterodine (Detrol, and generics) and solifenacin (Vesicare, and generics), reduce OAB symptoms by inhibiting involuntary bladder contractions and relaxing detrusor smooth muscle, but they can cause dry mouth and constipation, and their long-term use has been associated with a dose-related increase in the risk of dementia.2 Mirabegron appears to be similar in effectiveness to anticholinergic drugs and better tolerated, but it has been associated with small increases in blood pressure.3
Combination therapy with an anticholinergic drug and a beta-3 agonist can be considered when monotherapy is ineffective; mirabegron is FDA-approved for use both alone and in combination with solifenacin. Options for patients with refractory OAB include peripheral tibial nerve stimulation, sacral neuromodulation, and intra-detrusor injection of onabotulinumtoxinA (Botox).1,4
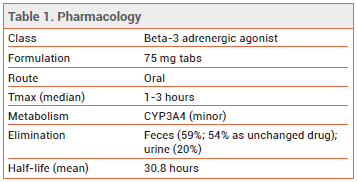
MECHANISM OF ACTION — Like mirabegron, vibegron activates beta-3 adrenergic receptors in the bladder, resulting in relaxation of detrusor smooth muscle during the storage phase of the fill-void cycle and increased bladder capacity. Although no direct comparisons are available, vibegron appears to be more selective than mirabegron in vitro for beta-3 receptors compared to beta-1 and beta-2 receptors.5,6
CLINICAL STUDIES — FDA approval of vibegron was based on the results of a 12-week, double-blind trial (EMPOWUR) in 1518 patients with symptomatic OAB who were randomized to receive once-daily treatment with vibegron 75 mg, tolterodine extended-release 4 mg (active control), or placebo. At 12 weeks, the reduction in mean daily micturitions, a coprimary endpoint, was significantly greater with vibegron, but not with tolterodine, compared to placebo (-1.8 with vibegron, -1.6 with tolterodine, and -1.3 with placebo). The reduction in mean daily urge urinary incontinence episodes, the other coprimary endpoint, was significantly greater with both vibegron and tolterodine than with placebo (-2.0 and -1.8 vs -1.4). Patients taking vibegron also had significant improvements, compared to those taking placebo, in volume voided per micturition, urgency episode frequency, and quality of life.7-9
ADVERSE EFFECTS — The most common adverse effects that occurred more frequently with vibegron than with placebo in EMPOWUR were headache (4.0% vs 2.4%) and nasopharyngitis (2.8% vs 1.7%). The incidence of hypertension was 1.7% in patients taking vibegron, 1.7% in the placebo group, and 2.6% in the tolterodine group. Urinary retention was reported in 0.6% of patients who took vibegron and in 0.4% of those who took placebo.7
PREGNANCY AND LACTATION — No data are available on the use of vibegron in pregnant or lactating women. No adverse effects were observed in the offspring of pregnant animals exposed to 89 times the level of vibegron recommended in humans. Radioactivity has been detected in the milk of lactating rats administered radiolabeled vibegron.
DRUG INTERACTIONS — Taking vibegron with an anticholinergic drug could increase the risk of urinary retention. Concomitant use of vibegron increased the Cmax and AUC of digoxin by 21% and 11%, respectively.
DOSAGE AND ADMINISTRATION — The recommended dosage of vibegron is 75 mg taken orally once daily with a glass of water. The tablets should either be swallowed whole or crushed and mixed with a tablespoonful of applesauce, and consumed immediately. Vibegron is not recommended for use in patients with end-stage renal disease (eGFR <15 mL/min/1.73 m2) or in those with severe hepatic impairment (Child-Pugh C).
CONCLUSION — The selective beta-3 adrenergic agonist vibegron (Gemtesa) was more effective than placebo and appeared to be similar in efficacy to the anticholinergic drug tolterodine for treatment of overactive bladder (OAB) in one 12-week trial. Unlike mirabegron (Myrbetriq), the other beta-3 agonist approved for treatment of OAB, vibegron has not been shown to increase blood pressure, but no direct comparisons of the two drugs are available. Behavioral modification is still preferred for initial treatment of OAB, but mirabegron and vibegron, which have few drug interactions and lack anticholinergic adverse effects, are reasonable choices for patients who require pharmacologic treatment.
- DJ Lightner et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment 2019. J Urol 2019; 202:558.
- SL Gray et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401.
- Mirabegron (Myrbetriq) for overactive bladder. Med Lett Drugs Ther 2013; 55:13.
- Botox for overactive bladder. Med Lett Drugs Ther 2013; 55:31.
- J Di Salvo et al. Pharmacological characterization of a novel beta 3 adrenergic agonist, vibegron: evaluation of antimuscarinic receptor selectivity for combination therapy for overactive bladder. J Pharmacol Exp Ther 2017; 360:346.
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Assessment report: Betmiga. Available at: https://bit.ly/31TDy1Z. Accessed April 15, 2021.
- D Staskin et al. International phase III, randomized, double-blind, placebo and active controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol 2020; 204:316.
- J Frankel et al. Vibegron improves quality-of-life measures in patients with overactive bladder: patient-reported outcomes from the EMPOWUR study. Int J Clin Pract 2020 December 17 (epub).
- S Varano et al. Efficacy and safety of once-daily vibegron for treatment of overactive bladder in patients aged ≥65 and ≥75 Years: subpopulation analysis from the EMPOWUR randomized, international, phase III Study. Drugs Aging 2021; 38:137.