ISSUE1687
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Review the recommendations for use of the updated 2023-2024 mRNA COVID-19 vaccines.
New 2023-2024 formulations of the mRNA COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) that more closely target currently circulating variants have been licensed by the FDA for use in persons ≥12 years old and made available under FDA Emergency Use Authorizations (EUAs) for use in persons 6 months to 11 years old. The bivalent (original and Omicron BA.4/5) formulations of the Pfizer and Moderna vaccines are no longer authorized for use in the US.1-3
THE NEW VACCINES – The new formulations are monovalent vaccines that code for the spike protein of the XBB.1.5 Omicron strain of SARS-CoV-2.
EFFICACY – No clinical studies evaluating the effectiveness of the new vaccine formulations are available. Licensure and authorization of both vaccines were based on safety and efficacy data with previous formulations of the vaccines and on immunogenicity data showing that the neutralization potency of the new formulations against currently circulating strains of the virus, including XBB.1.5, EG.5 (Eris), FL.1.5.1 (Fornax), and BA.2.86 (Pirola), is similar to that of previous formulations against the SARS-CoV-2 strains that they targeted.1,4,5
ADVERSE EFFECTS — Adverse effects of earlier versions of the mRNA COVID-19 vaccines have included injection-site reactions, fatigue, irritability, fever, chills, muscle and joint pain, headache, vomiting, decreased appetite, and lymphadenopathy. Severe allergic reactions and Guillain-Barré syndrome have been reported. Myocarditis and pericarditis can occur; the incidence is highest in adolescent and young adult males.6
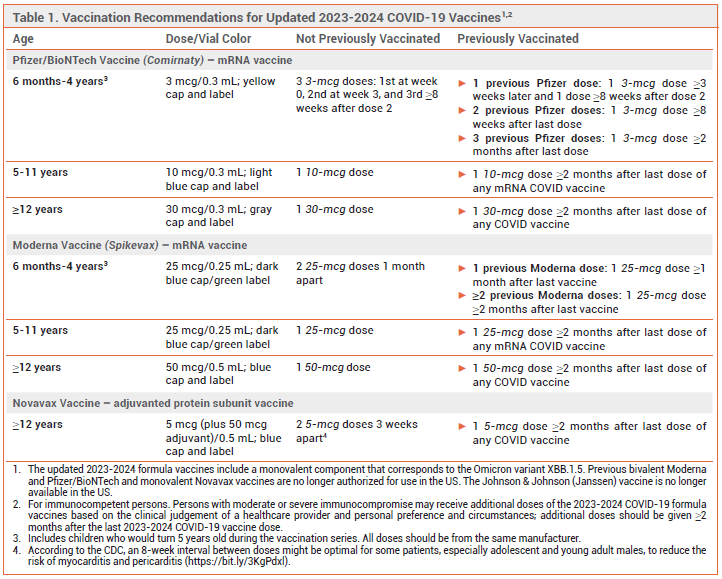
DOSAGE RECOMMENDATIONS – Generally, persons ≥5 years old should receive a single dose of a 2023-2024 vaccine formulation ≥2 months after their previous COVID-19 vaccine dose. Children 6 months to 4 years old who have already received ≥2 Pfizer or Moderna vaccine doses should receive a single dose of the corresponding 2023-2024 vaccine ≥8 weeks (Pfizer) or ≥2 months (Moderna) after their most recent dose. Those 6 months to 4 years old who received <2 previous doses should be given additional doses until they have received 3 total doses of the Pfizer vaccine or 2 total doses of the Moderna vaccine. Children 6 months to 11 years old with immunocompromise (solid-organ transplant recipients and equivalent) should receive at least 3 total age-appropriate COVID-19 vaccine doses, including at least 1 dose of a 2023-2024 formulation. Dosage recommendations for the updated vaccine formulations are summarized in Table 1.2,3
CDC RECOMMENDATIONS – The CDC recommends that all persons ≥6 months old receive either of the two 2023-2024 mRNA COVID-19 vaccines.7
- FDA News Release. FDA takes action on updated mRNA COVID-19 vaccines to better protect against currently circulating variants. September 11, 2023. Available at: https://bit.ly/3ZmmVcg. Accessed September 18, 2023.
- FDA. Fact sheet for healthcare providers administering vaccine: Emergency Use Authorization (EUA) of Pfizer-BioNTech COVID-19 vaccine (2023-2024 formula), for 6 months through 11 years of age. September 11, 2023. Available at: https://bit.ly/3EBwxpR. Accessed September 18, 2023.
- FDA. Fact sheet for healthcare providers administering vaccine: Emergency Use Authorization (EUA) of Moderna COVID-19 vaccine (2023-2024 formula), for individuals 6 months through 11 years of age. September 11, 2023. Available at: https://bit.ly/3LkJ9p4. Accessed September 18, 2023.
- K Modjarrad. Monovalent XBB.1.5 BNT162b2 COVID-19 vaccine: ACIP presentation. September 12, 2023. Available at: https://bit.ly/3sUrBdc. Accessed September 18, 2023.
- F Priddy. Safety and immunogenicity of Moderna COVID-19 vaccine (2023-2024 formula). Monovalent XBB.1.5 variant vaccine. ACIP. September 12, 2023. Available at: https://bit.ly/46ePxGD. Accessed September 18, 2023.
- CDC. Updated COVID-19 vaccine recommendations are now available. September 12, 2023. Available at: https://bit.ly/44S6RQs. Accessed September 18, 2023.
- CDC. CDC recommends updated COVID-19 vaccine for fall/winter virus season. September 12, 2023. Available at: https://bit.ly/3rhTRWD. Accessed September 18, 2023.