ISSUE1599
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of the fixed-dose combination of metformin, linagliptin, and empagliflozin (Trijardy XR) for treatment of type 2 diabetes.
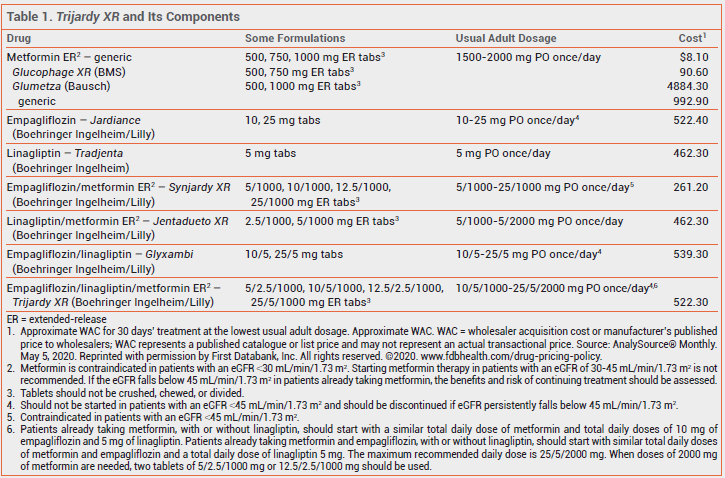
The FDA has approved Trijardy XR (Boehringer Ingelheim/Lilly), a fixed-dose combination of the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin,1 the dipeptidyl peptidase-4 (DPP-4) inhibitor linagliptin,2 and extended-release metformin, for oral treatment of type 2 diabetes in adults. Empagliflozin and linagliptin have been available in a fixed-dose combination as Glyxambi since 2015,3 and both have been available in 2-drug combinations with extended-release metformin for years (see Table 1).

Used alone, oral antihyperglycemic drugs usually lower glycated hemoglobin (A1C) by 0.5-1.5%. Metformin is generally the drug of choice for initial treatment of type 2 diabetes. If metformin alone does not achieve the desired A1C goal, comorbidities or cost may determine the choice of a second drug: options include an SGLT2 inhibitor or a GLP-1 receptor agonist for patients with cardiovascular disease or chronic kidney disease, an SGLT2 inhibitor for patients with heart failure, or a sulfonylurea if cost is an issue. If glycemic control is not achieved with maximum doses of 2 drugs, insulin or another drug can be added.4
Two randomized, open-label, crossover studies in a total of 60 healthy adults found that the 3-drug combination tablet was bioequivalent to individual tablets of empagliflozin, linagliptin, and extended-release metformin taken together.5
No new efficacy trials were required for approval of Trijardy XR. Approval of the 3-drug combination was based on the results of earlier trials in which addition of empagliflozin/linagliptin to metformin reduced A1C significantly more than addition of either drug alone.
The labeling of Trijardy XR includes a long list of warnings and precautions about adverse effects, including lactic acidosis associated with metformin, acute pancreatitis with DPP-4 inhibitors, and euglycemic ketoacidosis with SGLT2 inhibitors. As might be expected with a 3-drug combination available in 4 different strengths, the dosing instructions for starting treatment are complex.
- Empagliflozin (Jardiance) for diabetes. Med Lett Drugs Ther 2014; 56:99.
- Linagliptin (Tradjenta) – a new DPP-4 inhibitor for type 2 diabetes. Med Lett Drugs Ther 2011; 53:49.
- Glyxambi – a new combination for type 2 diabetes. Med Lett Drugs Ther 2015; 57:65.
- Drugs for type 2 diabetes. Med Lett Drugs Ther 2019; 61:169.
- I Lingvay et al. Triple fixed-dose combination of empagliflozin, linagliptin, and metformin for patients with type 2 diabetes. Postgrad Med 2020 May 4 (epub).