ONLY
ARTICLE
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of oritavancin (Kimyrsa) for skin and skin structure infections.
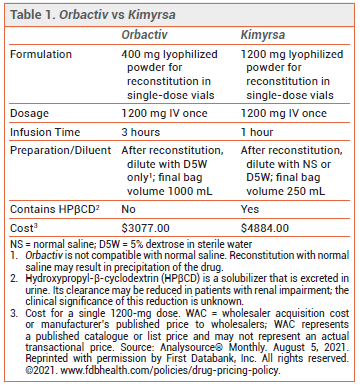
The FDA has approved Kimyrsa (Melinta), a new IV formulation of the long-acting lipoglycopeptide antibiotic oritavancin, for treatment of adults with acute bacterial skin and skin structure infections caused by susceptible gram-positive bacteria. Orbactiv (Melinta), another IV formulation of oritavancin, was approved in 2014 for the same indication.1 Kimyrsa has a smaller infusion volume (250 mL vs 1 L) and a shorter infusion time (1 hour vs 3 hours) compared to Orbactiv (see Table 1).
ACTIVITY — Oritavancin has demonstrated clinical activity against Staphylococcus aureus (including methicillin-resistant strains [MRSA]), Streptococcus agalactiae, Streptococcus anginosus group, Streptococcus dysgalactiae, Streptococcus pyogenes, and vancomycin-susceptible isolates of Enterococcus faecalis. The drug has been reported to have in vitro activity against vancomycin-susceptible and vancomycin-resistant isolates of Enterococcus faecium.2
CLINICAL STUDIES — FDA approval of Kimyrsa was based on the results of earlier clinical trials with Orbactiv3,4 and one pharmacokinetic study (summarized in the package insert) that showed the two formulations were bioequivalent.
ADVERSE EFFECTS — The most common adverse effects of oritavancin are headache, nausea, vomiting, limb and subcutaneous abscess, and diarrhea. Serious hypersensitivity reactions have been reported; in clinical trials, the median onset of hypersensitivity reactions was 1.2 days after administration and the median duration was 2.4 days. Patients with a history of hypersensitivity to another glycopeptide (vancomycin, telavancin, or dalbavancin) may be at increased risk. Infusion-related reactions such as pruritus, urticaria, and flushing have also been reported.
DRUG AND LABORATORY TEST INTERACTIONS — Oritavancin can interfere with coagulation tests by binding to and preventing the action of commonly used phospholipid reagents; it has no effect on coagulation in vivo. Use of IV unfractionated heparin is contraindicated for 120 hours after administration of oritavancin because the drug can falsely elevate activated partial thromboplastin time (aPTT). Oritavancin also artificially prolongs prothrombin time (PT) and INR for up to 12 hours after administration.
Oritavancin is a weak inhibitor of CYP2C9 and 2C19 and a weak inducer of CYP3A4 and 2D6; coadministration with drugs that are primarily metabolized by one of these enzymes can alter their serum concentrations.
CONCLUSION — Kimyrsa, a new formulation of the long-acting lipoglycopeptide antibiotic oritavancin, has an infusion time of 1 hour and an infusion volume of 250 mL, compared to 3 hours and 1 L with the original formulation (Orbactiv). It offers a more convenient option for treatment of adults with acute bacterial skin and skin structure infections, but it is more expensive
- Oritavancin (Orbactiv) for skin and skin structure infections. Med Lett Drugs Ther 2015; 57:3.
- A Belley et al. Comparative pharmacodynamics of single-dose oritavancin and daily high-dose daptomycin regimens against vancomycin-resistant Enterococcus faecium isolates in an in vitro pharmacokinetic/pharmacodynamic model of infection. Antimicrob Agents Chemother 2017; 61:e01265.
- GR Corey et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 2014; 370:2180.
- GR Corey et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis 2015; 60:254.