ISSUE1724
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Discuss the potential advantage of single-dose testosterone cypionate (Azmiro) over multidose formulations (Depo-Testosterone, and generics).
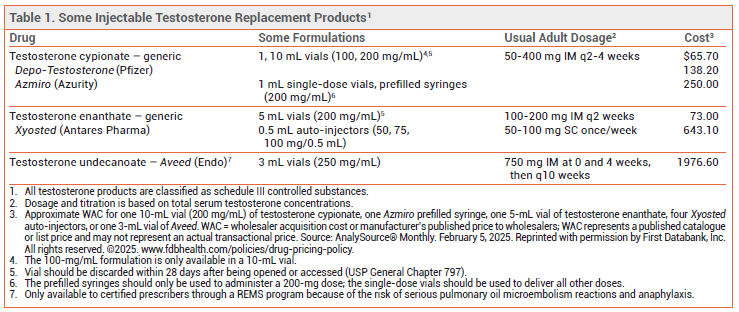
The FDA has approved Azmiro (Azurity), the first injectable formulation of testosterone cypionate to become available in single-dose vials and prefilled syringes for treatment of males with conditions associated with a deficiency or absence of endogenous testosterone. Injectable testosterone cypionate has been available in multidose vials (Depo-Testosterone, and generics) for many years. Testosterone enanthate (Xyosted) is available in prefilled autoinjectors for use in adult men.1 No testosterone products are approved for treatment of low testosterone levels due solely to aging. All testosterone products are classified as schedule III controlled substances.

THE NEW PRODUCT — Testosterone cypionate can crystallize in solution when it is stored at lower-than-recommended temperatures (less than 20-25°C; 68-77°F). According to its manufacturer, Azmiro is more stable than testosterone cypionate in multidose formulations and should not crystallize.2
CLINICAL STUDIES — FDA approval of Azmiro was based on the results of an open-label, single-dose, two-way crossover pharmacokinetic study (summarized in an FDA review document) in 28 hypogonadal men. The new single-dose prefilled testosterone cypionate formulation was bioequivalent to the multidose formulation of injectable testosterone cypionate (Depo-Testosterone).2
ADVERSE EFFECTS — The most common adverse effects of Azmiro in the single-dose study were mild injection-site reactions; the incidence was similar to that with Depo-Testosterone.
All testosterone products can cause erythrocytosis. Hematocrit levels should be assessed before starting treatment and periodically thereafter; testosterone therapy should be withheld for a first elevation until the hematocrit returns to an acceptable level and permanently discontinued for a second elevation.
Testosterone replacement therapy can cause venous thromboembolism, azoospermia, edema, gynecomastia, hypercalcemia, hepatic adverse effects, increases in blood pressure, and decreases in both HDL-cholesterol and thyroxine-binding globulin (TBG) levels. It has been associated with new-onset and worsening of sleep apnea, worsening of benign prostatic hyperplasia (BPH), and increases in prostate-specific antigen (PSA) levels; whether it increases the risk of prostate cancer is unclear.3 Like other testosterone products, Azmiro is contraindicated for use in patients with breast or prostate cancer.
DOSAGE AND ADMINISTRATION — Single-dose vials and prefilled syringes of Azmiro contain 200 mg of testosterone cypionate in 1 mL of solution. The recommended dosage is 50-400 mg administered by a healthcare provider every 2-4 weeks as a deep injection into the gluteal muscle. The prefilled syringes should only be used to administer a 200-mg dose; the single-dose vials can be used for all other doses.
CONCLUSION — Azmiro, a single-dose vial and prefilled syringe formulation of testosterone cypionate, is claimed to be more stable than testosterone cypionate multidose formulations (Depo-Testosterone, and generics) and should not crystallize. It is administered as a deep intragluteal injection by a healthcare provider every 2 to 4 weeks.
- Xyosted – a testosterone auto-injector for hypogonadism. Med Lett Drugs Ther 2019; 61:37.
- FDA, CDER. Multi-discipline review. Testosterone cypionate injection. June 2, 2022. Available at: https://bit.ly/3CCH6eO. Accessed February 27, 2025.
- Safety of testosterone replacement therapy. Med Lett Drugs Ther 2016; 58:33.