RELEASE
ARTICLE
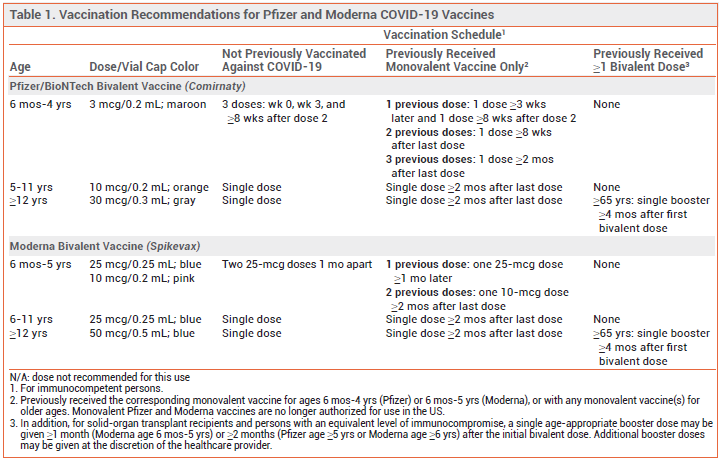
The FDA has amended its Emergency Use Authorizations (EUAs) for the bivalent mRNA COVID-19 vaccines (original and Omicron BA.4/5 strains) manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) to permit their use for all doses administered to persons ≥6 months old. The monovalent Pfizer and Moderna vaccines are no longer authorized for use in the US.1
All persons ≥6 months old who completed a primary series with the Pfizer or Moderna monovalent vaccine may now receive a single dose of bivalent vaccine; an additional bivalent dose can generally be given to persons ≥65 years old and to those with significant immunocompromise. For unvaccinated persons ≥5 years old (Pfizer) or ≥6 years old (Moderna), a single bivalent dose is now recommended for primary immunization; multiple doses are recommended for younger children. The new recommendations for the Pfizer and Moderna vaccines are summarized in Table 1.1-3
- FDA News Release. Coronavirus (COVID-19) update: FDA authorizes changes to simplify use of bivalent mRNA COVID-19 vaccines. April 18, 2023. Available at: https://bit.ly/3A9lguO. Accessed May 1, 2023.
- FDA. Pfizer-BioNTech COVID-19 vaccines. Pfizer-BioNTech COVID-19 vaccine, bivalent now authorized for all doses. April 18, 2023. Available at: https://bit.ly/3Mz59fj. Accessed May 1, 2023.
- FDA. Moderna COVID-19 vaccines. Moderna COVID-19 vaccine, bivalent now authorized for all doses. April 18, 2023. Available at: https://bit.ly/3MwVyWk. Accessed May 1, 2023.