RELEASE
ARTICLE
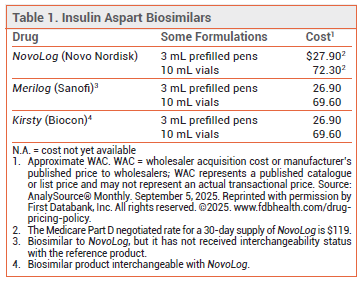
The FDA has approved Kirsty (Biocon), a biosimilar to rapid-acting insulin aspart (NovoLog), for treatment of type 1 or type 2 diabetes. Kirsty is the first rapid-acting insulin to become available in the US that has received interchangeability status with NovoLog; Merilog, another insulin aspart biosimilar, was approved earlier but has not received interchangeability status with NovoLog.1

REGULATORY STATUS — A biosimilar is a biologic product that is highly similar in composition, strength, and biological properties and has no clinically meaningful differences in efficacy, safety, purity, and potency to the FDA-approved reference product. For a biosimilar to be approved as an interchangeable product, the manufacturer must conduct clinical trials to prove that the results will be the same if the patient switches between the reference product and the biosimilar.2
Under federal law, an interchangeable product can be substituted for the reference product by the pharmacist without permission from the prescriber. Some states require the pharmacist to notify the prescriber and/or patient before making the substitution. Currently four states (AL, IN, SC, and WA) do not permit interchangeability under any circumstances.3
CLINICAL STUDIES — FDA approval of Kirsty was based on the results of an open-label trial in 478 patients with type 1 diabetes. Patients were randomized to receive mealtime Kirsty or NovoLog, each in addition to once-daily, long-acting insulin glargine (Lantus). The mean decrease in A1C levels from baseline to 26 weeks with Kirsty was noninferior to that with NovoLog (-0.10% with Kirsty vs -0.04% with NovoLog). Fasting plasma glucose, postprandial glucose excursions, and adverse effects were also similar between the two groups.4
DOSAGE AND ADMINISTRATION — Like NovoLog, Kirsty is supplied in 3-mL prefilled pens and 10-mL multidose vials containing 100 units/mL of insulin aspart. Insulin aspart should be administered subcutaneously into the abdomen, thigh, buttocks, or upper arm 5-10 minutes before a meal. The injection site should be rotated to minimize the risk of lipodystrophy and cutaneous amyloidosis.
CONCLUSION — The biosimilar insulin aspart Kirsty can be substituted for NovoLog by the pharmacist without permission from the prescriber. Kirsty will presumably be less expensive than NovoLog.
- In brief: Merilog – a NovoLog biosimilar. Med Lett Drugs Ther 2025; 67:104.
- FDA Guidance Document. Clinical immunogenicity considerations for biosimilar and interchangeable insulin products. November 2019. Available at: https://bit.ly/3Hgo2Fw. Accessed September 5, 2025.
- S Jeremias. Part 2: for patients and employers, 2023 means a changed landscape. AJMC The Center for Biosimilars. September 13, 2022. Available at: https://bit.ly/41mbFyw. Accessed September 5, 2025.
- TC Blevins et al. Immunogenicity, efficacy, and safety of biosimilar insulin aspart (MYL-1601D) compared with originator insulin aspart (Novolog) in patients with type 1 diabetes after 24 weeks: a randomized open-label study. BioDrugs 2022; 36:761. doi:10.1007/s40259-022-00554-6
