RELEASE
ARTICLE
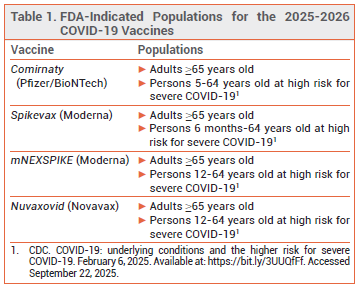
The FDA has licensed new 2025-2026 formulations of the mRNA COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax, mNEXSPIKE1) and the adjuvanted protein subunit COVID-19 vaccine manufactured by Novavax (Nuvaxovid). The new formulations are indicated for use in all adults ≥65 years old and in persons 6 months (Spikevax), 5 years (Comirnaty), or 12 years (mNEXSPIKE, Nuvaxovid) through 64 years old who are at high risk for severe COVID-19 because of an underlying condition.2 An Emergency Use Authorization allowing administration of the Pfizer vaccine to children 6 months through 4 years old has been withdrawn.
THE NEW FORMULATIONS — The 2025-2026 formulations of Comirnaty and Spikevax code for the entire spike protein of the LP.8.1 Omicron strain of SARS-CoV-2; mNEXSPIKE codes only for the N-terminal and receptor-binding domains of the LP.8.1 spike protein.1 Nuvaxovid contains the spike protein of the JN.1 Omicron strain of the virus. The XFG Omicron strain of SARS-CoV-2, which was the most prevalent variant in US wastewater as of September 2025,3 and the LP.8.1 strain are both descended from the JN.1 strain.4
EFFICACY — No clinical studies evaluating the immunogenicity or eff cacy of the new vaccine formulations were required. Licensure of the vaccines was based on the immunogenicity, safety, and efficacy of previous vaccine formulations.
Last Season – The efficacy of the 2024-2025 COVID-19 vaccine formulations was evaluated in an interim case-control analysis of emergency department and urgent care (ED/UC) visits and hospitalizations occurring between September 2024 and January 2025 that were reported in a large electronic health record (EHR-based network (VISION). Among 137,543 adults who had an ED/UC visit, receipt of a 2024-2025 COVID-19 vaccine within the previous 7-119 days was associated with a reduced risk of a COVID-19-associated visit; the estimated vaccine efficacy was 33% (30% in adults 18-64 years old and 36% in those ≥65 years old). Among adults ≥65 years old who were hospitalized, receipt of a 2024-2025 vaccine 7-119 days previously was associated with a reduced risk of a subsequent COVID-19-associated hospitalization; the estimated vaccine efficacy was 45% in immunocompetent persons (n=26,219) and 40% in immunocompromised persons (n=8192).5
Similar results were observed in an analysis of a different EHR-based network (IVY); among 1929 immunocompetent adults ≥65 years old who were hospitalized during the same time period, those who had received a 2024-2025 vaccine 7-119 days previously had a lower risk of a COVID-19-associated hospitalization (estimated vaccine efficacy 46%).5
ADVERSE EFFECTS — Adverse effects of previous formulations of COVID-19 vaccines have included injection-site reactions, fatigue, irritability, fever, chills, muscle and joint pain, headache, vomiting, decreased appetite, and lymphadenopathy. Severe allergic reactions and Guillain-Barré syndrome have been reported. Myocarditis and pericarditis can occur; the incidence is highest in adolescent and young adult males.6,7
PREGNANCY AND LACTATION — Pregnancy has been associated with an increased risk of COVID-19 complications, including pneumonia, intensive care unit admission, intubation, and death.8,9 Vaccination protects pregnant women against COVID-19 complications and provides passive immunity to their infants for the first few months after birth.10,11 Administration of COVID-19 vaccines to nursing mothers is considered safe.12 The American College of Obstetricians and Gynecologists has recommended that all pregnant and breastfeeding women be vaccinated against COVID-19.10
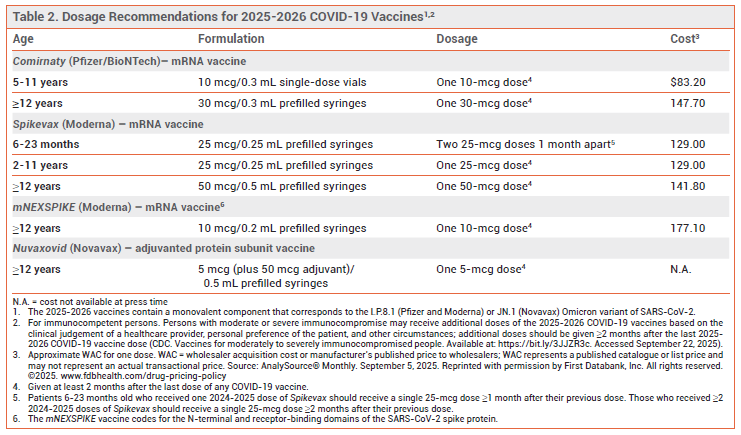
DOSAGE AND ADMINISTRATION — For most patients, a single dose of a 2025-2026 COVID-19 vaccine administered ≥2 months after any previous COVID-19 vaccine dose is recommended. Additional doses, each given ≥2 months after the previous dose, can be considered for persons with immunocompromise (solid-organ transplant recipients and equivalent).13 Children 6-23 months old who have never been vaccinated against COVID-19 should receive two doses of Spikevax 1 month apart (see Table 2).
RECOMMENDATIONS — The recently restructured CDC Advisory Committee on Immunization Practices (ACIP) has recommended that administration of 2025-2026 COVID-19 vaccines to persons ≥6 months old be based on shared clinical decision-making. It has emphasized that, for persons <65 years old, the benefits of vaccination against COVID-19 most greatly outweigh the risks in those who are at elevated risk for severe disease.14
The American Academy of Family Physicians (AAFP) recommends that all adults, including pregnant and lactating women, and all children 6-23 months old receive a COVID-19 vaccine this season. They recommend vaccination of persons 2-18 years old who are at increased risk of severe COVID-19.15
The American Academy of Pediatrics (AAP) has recommended that all children 6-23 months old and all children who have never been vaccinated against COVID-19 receive a 2025-2026 vaccine (not just those with risk factors for severe disease); it also recommends vaccination of children who reside in long-term care facilities or other congregate settings and those with household contacts who are at high risk for severe COVID-19.12
The American College of Obstetricians and Gynecologists (ACOG) has recommended that all pregnant and breastfeeding women be vaccinated against COVID-19.16
- COVID-19 update: mNEXSPIKE – a new Moderna mRNA vaccine for COVID-19. Med Lett Drugs Ther 2025; 67:119.
- CDC. COVID-19: underlying conditions and the higher risk for severe COVID-19. February 6, 2025. Available at: https://bit.ly/3UUQfFf. Accessed September 22, 2025.
- CDC. National Wastewater Surveillance System (NWSS): COVID-19 variants in wastewater. Available at: https://bit.ly/47WhYgq. Accessed September 22, 2025.
- Nextstrain. Genomic epidemiology of SARS-CoV-2 with subsampling focused globally over the past 6 months. September 21, 2025. Available at: https://bit.ly/4lXsxTH. Accessed September 22, 2025.
- R Link-Gelles et al. Interim estimates of 2024-2025 COVID-19 vaccine effectiveness among adults aged ≥18 years – VISION and IVY networks, September 2024-January 2025. MMWR Morb Mortal Wkly Rep 2025; 74:73. doi:10.15585/mmwr.mm7406a1
- CDC. Clinical considerations: myocarditis and pericarditis after receipt of COVID-19 vaccines among adolescents and young adults. October 10, 2023. Available at: https://bit.ly/3yXicot. Accessed September 22, 2025.
- COVID-19 update: mRNA COVID-19 vaccines and myocarditis. Med Lett Drugs Ther 2025; 67:119.
- LD Zambrano et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status – United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1641. doi:10.15585/mmwr.mm6944e3
- RJ Martinez-Portilla et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx). Ultrasound Obstet Gynecol 2021; 57:224. doi:10.1002/uog.23575
- American College of Obstetricians and Gynecologists. COVID-19 vaccination considerations for obstetric-gynecologic care. September 2025. Available at: https://bit.ly/4g3oHY4. Accessed September 22, 2025.
- LL Shook et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA 2022; 327:1087. doi:10.1001/jama.2022.1206
- Drugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. COVID-19 vaccines. [Updated 2025 August 15]. Available at: https://bit.ly/3Sv9dkG. Accessed September 22, 2025.
- CDC. COVID-19: vaccines for moderately to severely immunocompromised people. June 11, 2025. Available at: https://bit.ly/3JJZR3c. Accessed September 22, 2025.
- HHS. ACIP recommends COVID-19 immunization based on individual decision-making. September 19, 2025. Available at: https://bit.ly/46sbeVb. Accessed September 22, 2025.
- AAFP. AAFP announces fall immunization recommendations, reaffirming commitment to vaccine safety and public health. September 8, 2025. Available at: https://bit.ly/3K0wSZa. Accessed September 22, 2025.
- American Academy of Pediatrics Committee on Infectious Diseases. Recommendations for COVID-19 vaccines in infants, children, and adolescents: policy statement. Pediatrics 2025 (epub). doi:10.1542/peds.2025-073924