RELEASE
ARTICLE
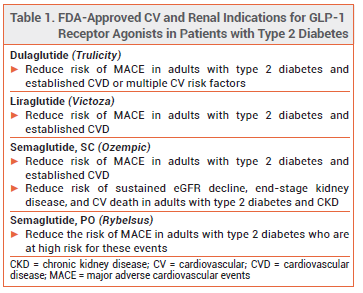
The oral glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Rybelsus – Novo Nordisk), which was approved by the FDA in 2019 for treatment of type 2 diabetes in adults, has now also been approved to reduce the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes who are at high risk for these events.1 The injectable GLP-1 receptor agonists semaglutide (Ozempic), dulaglutide (Trulicity), and liraglutide (Victoza) are also approved for cardiovascular risk reduction in patients with type 2 diabetes (see Table 1).2
CLINICAL STUDIES — FDA approval of oral semaglutide for the new indication was based on the results of a double-blind trial (SOUL) in 9650 patients ≥50 years old with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD), chronic kidney disease (CKD), or both. Patients were randomized to receive oral semaglutide titrated to 14 mg once daily or placebo in addition to standard care (other glucose-lowering and cardiovascular risk-reducing drugs). After a median of 49.5 months, the incidence of MACE (a composite of nonfatal myocardial infarction [MI]), nonfatal stroke, or cardiovascular death), the primary endpoint, was statistically significantly lower in the semaglutide arm compared to the placebo arm (12.0% vs 13.8%; HR 0.86, 95% CI 0.77-0.96 [number needed to treat 55.6]). The reduction was primarily driven by a reduction in nonfatal MI (HR 0.74, 95% CI 0.61-0.89). Cardiovascular mortality and adverse kidney outcomes were similar in both groups.3
DOSAGE, ADMINISTRATION, AND COST — Rybelsus is available in two formulations (R1 and R2). The R1 formulation, which is available in 3-, 7-, and 14-mg tablets, should be used for the new indication. Slow titration of the dose can reduce adverse GI effects and improve tolerability. The recommended starting dosage is 3 mg once daily for 30 days; the dose should be increased to 7 mg once daily for 60 days and, if needed for glycemic control, to 14 mg once daily thereafter. All patients in the SOUL trial were titrated to 14 mg. Rybelsus should be taken on an empty stomach in the morning with no more than 4 ounces of plain water. The wholesale acquisition cost (WAC) for a 30-day supply of Rybelsus is $997.60.4
CONCLUSION — Addition of the oral glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Rybelsus) to standard treatment reduced the risk of a major adverse cardiovascular event (MACE) in adults with type 2 diabetes at increased risks for these events. Rybelsus has not been directly compared with injectable GLP-1 receptor agonists for this indication.
View the Comparison Table: GLP-1 and GIP/GLP-1 Receptor Agonists for Type 2 Diabetes
- Oral semaglutide (Rybelsus) for type 2 diabetes. Med Lett Drugs Ther 2019; 61:166.
- Non-insulin drugs for type 2 diabetes. Med Lett Drugs Ther 2025 in press.
- DK McGuire et al. Oral semaglutide and cardiovascular outcomes in high-risk type 2 diabetes. N Engl J Med 2025; 392:2001. doi:10.1056/nejmoa2501006
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. October 5, 2025. Reprinted with permission by First Databank, Inc. All rights reserved. ©2025. www.fdbhealth.com/policies/drug-pricing-policy.