RELEASE
ARTICLE
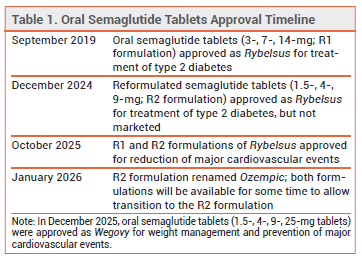
Oral semaglutide 1.5-, 4-, and 9-mg tablets, which were previously approved by the FDA (but never marketed) as the R2 formulation of Rybelsus, have now been approved as Ozempic (see Table 1). Both the original R1 formulation of Rybelsus (3-, 7-, and 14-mg tablets) and the renamed Ozempic tablets are FDA-approved for treatment of type 2 diabetes and to reduce the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes who are at risk for these events.1,2 The R1 (Rybelsus) and R2 (Ozempic) formulations are not interchangeable on a mg-per-mg basis; Ozempic tablets contain inactive ingredients that enhance drug absorption and have greater bioavailability than Rybelsus R1 tablets.
The name change capitalizes on the brand-name recognition of Ozempic, which has been available for subcutaneous administration in patients with type 2 diabetes since 2017.
Ozempic tablets are expected to be available in the second quarter of 2026. Rybelsus R1 tablets will remain available for some time to allow for patients to switch to Ozempic tablets.