ISSUE1539
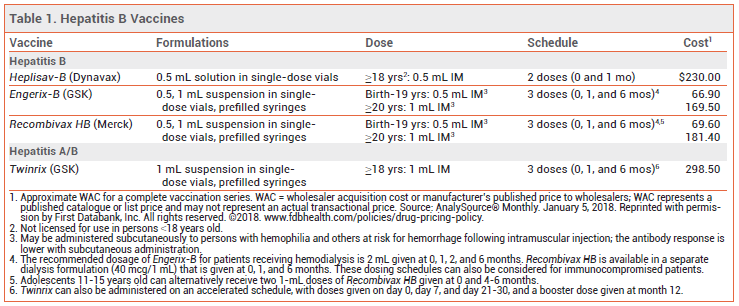
The FDA has approved a two-dose hepatitis B virus (HBV) vaccine (Heplisav-B – Dynavax) for use in adults ≥18 years old. The three other HBV vaccines marketed in the US are usually administered in 3 doses. Engerix-B and Recombivax HB are licensed for use in persons of all ages. A combination hepatitis A/B vaccine (Twinrix) contains the same hepatitis B component as Engerix-B and is licensed for use only in adults.1

HEPATITIS B VIRUS INFECTION — HBV infection is transmitted through percutaneous or mucosal contact with infectious blood or other bodily fluids. Risk factors for acquisition in adults include occupational exposure, IV drug abuse, unprotected sex, and hemodialysis. Chronic HBV infection can cause cirrhosis and hepatic cancer. Universal childhood vaccination against HBV, introduced in the US in 1991, has significantly reduced the incidence of HBV infection.2
THE VACCINES — All four HBV vaccines available in the US (see Table 1) contain recombinant yeast-derived hepatitis B surface antigen (HBsAg) with an immunostimulatory adjuvant. Engerix-B, Recombivax HB, and Twinrix use aluminum hydroxide as an adjuvant. Heplisav-B uses a synthetic cytosine phosphoguanine oligonucleotide (CpG 1018) derived from bacterial DNA; it is thought to stimulate the immune system through activation of the toll-like receptor 9 (TLR-9) pathway, which induces production of cytokines such as interleukin-12 and interferon-alpha.3
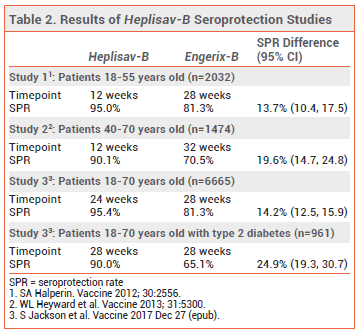
CLINICAL STUDIES — The immunogenicity of the new vaccine was evaluated in three randomized, observer-blinded studies that compared the rates of seroprotection (defined as an HBsAg anti body concentration ≥10 mIU/mL) after two doses of Heplisav-B given at 0 and 4 weeks to those after 3 doses of Engerix-B given at 0, 1, and 6 months. Seroprotection rates were significantly higher with Heplisav-B than with Engerix-B (see Table 2).4-6
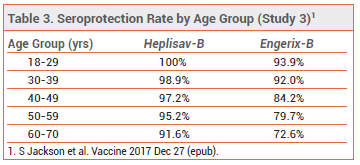
One of the studies evaluated seroprotection rates by age group; the immune response to both vaccines decreased with age, but seroprotection rates in all prespecified age groups were significantly higher with Heplisav-B than with Engerix-B (see Table 3).6
ADVERSE EFFECTS — The most common adverse effects of Heplisav-B in clinical trials were injection-site pain (23-39%), fatigue (11-17%), and headache (8-17%). Injection-site pain, redness, and swelling occurred more often with Heplisav-B than with Engerix-B, but the reported rates of death and serious adverse events with the two vaccines were similar.
RECOMMENDATIONS FOR ADULT IMMUNIZATION — Any person who wants protection against HBV infection should be immunized; no risk factor needs to be identified for vaccination to be indicated.
Hepatitis B immunization is specifically recommended for adults with a medical, occupational, or behavioral risk factor for HBV acquisition. Medical indications include chronic liver disease, end-stage renal disease and hemodialysis (Heplisav-B has not been studied in hemodialysis patients), diabetes (particularly in persons 19-59 years old), and HIV infection. Occupational indications include healthcare or public safety work with potential exposure to blood or bodily fluids. Adults with behavioral risks include injection drug users and those who have had multiple sex partners in the previous 6 months or recently acquired another sexually transmitted infection.
Other adult populations that should be vaccinated against HBV infection include men who have sex with men, residents of facilities for the aged and chronically ill, staff and clients of facilities that test for and treat sexually transmitted infections or drug abuse, residents and staff of institutions for the developmentally disabled, inmates and staff of correctional facilities, household contacts and sex partners of persons with chronic HBV infection, and travelers to countries with intermediate or high rates of chronic HBV infection.7,8
CONCLUSION — Heplisav-B, a new hepatitis B virus vaccine with a synthetic oligonucleotide immunostimulatory adjuvant, is licensed for use in adults ≥18 years old. In clinical trials, two doses of Heplisav-B were more immunogenic than three doses of an older hepatitis B virus vaccine (Engerix-B), but Heplisav-B caused more injection-site reactions. The rates of serious adverse effects with the two vaccines were similar, but the long-term safety of Heplisav-B remains to be established.
- Adult immunization. Treat Guidel Med Lett 2014; 12:39.
- CDC. Surveillance for viral hepatitis – United States, 2015. Available at: www.cdc.gov. Accessed January 18, 2018.
- J Scheiermann and DM Klinman. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014; 32:6377.
- SA Halperin et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine 2012; 30:2556.
- WL Heyward et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40–70 years of age. Vaccine 2013; 31:5300.
- S Jackson et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine 2017 Dec 27 (epub).
- S Schillie et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018; 67:1.
- Vaccines for travelers. Med Lett Drugs Ther 2014; 56:115.