ISSUE1615
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of the Pfizer-BioNTech COVID-19 vaccine.
May 11, 2021: New information about the Pfizer/BioNTech vaccine has been released since original posting of this article. Please click here to read the latest news.
Revised 6/15/21: The Storage and Administration section has been updated.
- Clinical Study
- Adverse Effects
- Pregnancy and Lactation
- Storage and Administration
- Immunization Priority
- References
Table
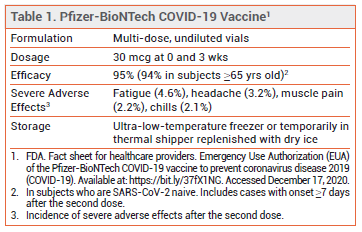
The FDA has issued an Emergency Use Authorization (EUA) for the Pfizer-BioNTech mRNA-based vaccine for prevention of COVID-19 in persons ≥16 years old.
CLINICAL STUDY — Issuance of the EUA was based primarily on the results of a double-blind trial in which 43,548 subjects ≥16 years old were randomized 1:1 to receive 30 mcg of the Pfizer-BioNTech vaccine (BNT162b2) or placebo at 0 and 3 weeks. There were 8 cases of COVID-19 with onset ≥7 days after the second dose among SARS-CoV-2-naive subjects who received the vaccine and 162 cases among those who received placebo; the vaccine efficacy rate was 95%. In adults ≥65 years old, the vaccine efficacy rate was 94%. Severe COVID-19 occurred after the first dose in 1 subject who received the vaccine and in 9 of those who received placebo.1
ADVERSE EFFECTS — Injection-site pain, fatigue, chills, headache, muscle and joint pain, fever, and diarrhea were common following administration of the vaccine. Most adverse effects were mild or moderate in severity and occurred at a higher frequency after the second dose. Older adults generally reported fewer and milder adverse reactions than younger vaccine recipients. The most commonly reported severe adverse effects were fatigue, headache, muscle pain, and chills.1
Lymphadenopathy occurred more often in those who received the vaccine (0.3% vs <0.1% with placebo). Four cases of Bell's palsy were reported in the vaccine group; a causal relationship has not been established.
Cases of anaphylaxis and anaphylactoid reactions to the Pfizer-BioNTech and Moderna COVID-19 vaccines have been reported; a CDC analysis of adverse effects following administration of ~1.9 million first doses of the Pfizer-BioNTech vaccine found the rate of anaphylaxis to be 11.1 per million doses.2 Experts have theorized that polyethylene glycol (PEG), which is present in both vaccines, may be the cause of these reactions. Both vaccines are contraindicated for use in persons with a history of immediate or severe allergic reaction to a previous dose of an mRNA vaccine or any of its components, including PEG; a history of allergy to polysorbate, which is structurally related to PEG, is a precaution to use of a mRNA COVID-19 vaccine. Appropriate medical treatment used to manage allergic reactions must be available for use following administration of an mRNA COVID-19 vaccine. Vaccine providers should observe persons with any history of immediate allergic reaction to a vaccine or injectable therapy or any history of anaphylaxis for 30 minutes after vaccination; other persons should be observed for 15 minutes after vaccination.8
PREGNANCY AND LACTATION — Pregnant women with COVID-19 are at increased risk for morbidity and mortality. According to the FDA, data on the Pfizer-BioNTech vaccine are insufficient to inform vaccine-associated risk in pregnancy. Data on the effects of the vaccine on the breastfed infant or on milk production are not available.3 The American College of Obstetricians and Gynecologists (ACOG) recommends that the vaccine not be withheld from pregnant or lactating women who are eligible for vaccination.4
STORAGE AND ADMINISTRATION — The Pfizer-BioNTech vaccine is supplied in frozen multi-dose vials. In the US, Pfizer is delivering the vaccine directly to points of use in temperature-controlled thermal shippers. Upon arrival, the vials should be transferred from the container to an ultralow- temperature freezer (-80°C to -60°C). Alternatively, the vials can be left in the thermal shippers as described in the guidelines packed in the containers, with dry ice periodically replenished to maintain a storage temperature of -96°C to -60°C. If necessary, vials can be transferred to a standard freezer (-25°C to -15°C) for a cumulative time of ≤2 weeks before being returned to the ultra-low-temperature freezer.
Before preparation and administration, the vaccine can be thawed in a standard refrigerator for up to one month or at room temperature for ≤2 hours; doses cannot be refrozen once thawed. The contents of each vial must then be diluted with 1.8 mL of normal saline to allow for administration of six 0.3- mL doses. A low dead-volume syringe is generally required to extract 6 full doses from a vial; residual vaccine from multiple vials should not be combined to form a full dose. Once diluted, the product must be stored at 2-25°C and used within 6 hours. Vials must be protected from light at all times before use.3,5
IMMUNIZATION PRIORITY — The CDC Advisory Committee on Immunization Practices (ACIP) recommends that healthcare personnel and long-term care facility residents be immunized first. Further recommendations about vaccine priority will be finalized at future ACIP meetings.6
The CDC has required state and local jurisdictions to develop vaccination plans for various phases of supply availability. Vaccines will generally be allocated to states and other jurisdictions based on population. State executives and health departments will be responsible for interpreting ACIP guidance and determining where the vaccine should be shipped and who should receive it.7
- FP Polack et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020 Dec 10 (epub).
- CDC. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine — United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep 2021 January 6 (epub).
- FDA. Fact sheet for health care providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Available at: https://bit.ly/37fX1NG. Accessed December 17, 2020.
- ACOG. Vaccinating pregnant and lactating patients against COVID-19. December 13, 2020. Available at: http://bit.ly/2Kt7AnS. Accessed December 17, 2020.
- Pfizer. Pfizer-BioNTech COVID-19 vaccine U.S. distribution fact sheet. November 20, 2020. Available at: https://bit.ly/37bL6zw. Accessed December 17, 2020.
- CDC Advisory Committee on Immunization Practices (ACIP). ACIP presentation slides: December 2020 meeting. December 1, 2020. Available at: https://bit.ly/37wZx1f. Accessed December 17, 2020.
- CDC. COVID-19 vaccination program interim playbook for jurisdiction operations – October 29, 2020. Version 2.0. Available at: https://bit.ly/37SIp63. Accessed December 17, 2020.
- CDC. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. January 6, 2021. Available at: http://bit.ly/38i7CIH. Accessed January 7, 2021.