ISSUE1547
The FDA has approved a lower-dose epinephrine auto-injector (Auvi-Q 0.1 mg – Kaléo) for emergency treatment of anaphylaxis in children weighing 7.5-15 kg (16.5-33 lbs). It is the first epinephrine auto-injector to be approved for use in infants and toddlers weighing less than 15 kg. Previously, Auvi-Q and other epinephrine auto-injectors were only available in 0.15- and 0.3-mg strengths for patients weighing 15-30 kg or ≥30 kg, respectively.1
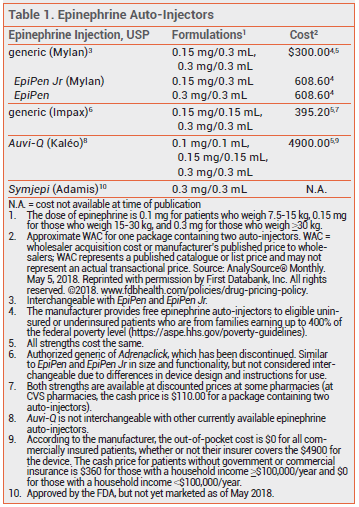
The recommended dose of epinephrine for intramuscular (IM) or subcutaneous (SC) administration is 0.01 mg/kg. None of the previously available epinephrine auto-injectors provided a weight-appropriate dose for infants, so many physicians prescribed a 0.15-mg auto-injector off-label for this age group.2
The Auvi-Q device is about the length and width of a credit card and as thick as a cell phone. It has an automatic needle retraction system and a red safety guard at the needle-end of the device. Removal of the outer case initiates visual signals and an audio recording that provides step-by-step instructions and a 2-second countdown during the injection process.
Auvi-Q should be injected IM into the anterolateral aspect of the thigh (through clothing, if necessary). After treatment with epinephrine, the patient should be taken to the nearest emergency department; anaphylaxis symptoms recur in up to 15% of patients hours after resolution of the initial symptoms.3
The needle length in the new 0.1-mg auto-injector is shorter than in other epinephrine auto-injectors. Use of a shorter needle decreases the risk of striking bone when administering a dose to a small child, but may result in SC rather than IM injection.4,5 Higher levels of epinephrine are obtained with IM injection than with SC injection.6
Auvi-Q 0.1 mg is supplied in a carton containing two single-use auto-injectors and a training device without a needle. The outer case protects the epinephrine solution from light; exposure to excessive heat or cold should be avoided. The shelf-life of the epinephrine in the auto-injector is 18 months. All three strengths of Auvi-Q are priced the same.
- Drugs for allergic disorders. Med Lett Drugs Ther 2017; 59:71.
- SH Sicherer and FER Simons et al. Epinephrine for first-aid management of anaphylaxis. Pediatrics 2017; 139:e20164006.
- S Lee et al. Update on biphasic anaphylaxis. Curr Opin Allergy Clin Immunol 2016; 16:346.
- H Kim et al. Inadequacy of current pediatric epinephrine autoinjector needle length for use in infants and toddlers. Ann Allergy Asthma Immunol 2017; 118:719.
- S Dreborg et al. Epinephrine auto-injector needle lengths: can both subcutaneous and periosteal/intraosseous injection be avoided? Ann Allergy Asthma Immunol 2018 Feb 27 (epub).
- FE Simons et al. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101:33.
