ISSUE1612
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Discuss the efficacy and safety of remdesivir (Veklury) for treatment of COVID-19.
- Mechanism of Action
- Clinical Studies
- Timing of Administration
- Adverse Effects
- Pregnancy and Lactation
- Drug Interactions
- Dosage, Administration, and Cost
- Conclusion
- References
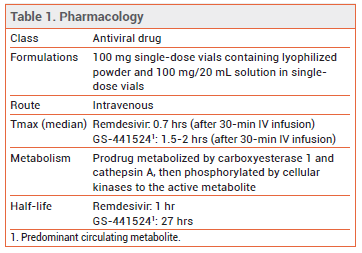
Table
The FDA has approved the antiviral drug remdesivir (Veklury – Gilead) for IV treatment of COVID-19 in hospitalized patients who are ≥12 years old and weigh ≥40 kg. Hospitalized children who are <12 years old or weigh <40 kg can receive remdesivir through an Emergency Use Authorization (EUA). Remdesivir is the first drug to be approved in the US for treatment of COVID-19.

MECHANISM OF ACTION — Remdesivir is a nucleotide prodrug of an adenosine analog that inhibits viral RNA-dependent RNA polymerase. It is active against SARS-CoV-2 and some other coronaviruses in vitro and in animal models.
CLINICAL STUDIES — FDA approval of remdesivir was based on the results of three randomized controlled trials in hospitalized patients with COVID-19.
In a double-blind trial (ACTT-1), 1062 hospitalized adults with COVID-19 and evidence of lower respiratory tract infection were randomized to receive remdesivir or placebo for up to 10 days, in addition to standard treatment. About 15% of patients had mild to moderate disease and 85% had severe disease. Median time to recovery within 29 days of receiving treatment, defined as being discharged or still hospitalized but no longer requiring supplemental oxygen, the primary endpoint, was shorter with remdesivir than with placebo (10 vs 15 days; RR 1.29, 95% CI 1.12 to 1.49). The Kaplan-Meier estimate of mortality by day 29 was 11.4% with remdesivir versus 15.2% with placebo (HR 0.73, 95% CI 0.52 to 1.03).1
In an open-label trial (SIMPLE-Moderate), 584 hospitalized adults with moderate COVID-19 (pneumonia and room-air oxygen saturation >94%) were randomized to receive remdesivir for either 5 or 10 days or standard treatment. Patients who received remdesivir for 5 days, but not those who received it for 10 days, were more likely to have an improvement in their clinical status on day 11 than those who received standard treatment (odds ratio 1.65; 95% CI 1.09-2.48). All-cause mortality at day 28 was ≤2% in all 3 groups.2
In another open-label trial (SIMPLE-Severe), 397 hospitalized adults with severe COVID-19 (pneumonia and oxygen saturation of ≤94% or requiring supplemental oxygen) were randomized to receive remdesivir for either 5 or 10 days. By day 14, a clinical improvement of ≥2 points on a 7-point ordinal scale, the primary endpoint, occurred in 64% of patients treated with remdesivir for 5 days compared to 54% of those treated for 10 days, a statistically significant difference. In the 5-day treatment group, discharge rates were higher (60% vs 52%), particularly among those who had symptoms for <10 days before receiving their first remdesivir dose, and mortality rates were lower (8% vs 11%), but the differences were not statistically significant. All-cause mortality at day 28 was 12% in the 5-day treatment group versus 14% in the 10-day treatment group.3
In a third open-label trial (WHO Solidarity), 11,266 hospitalized adults with COVID-19 were randomized to receive remdesivir, hydroxychloroquine, lopinavir/ritonavir, or interferon beta. Controls were local standard of care or one of the other study drugs. An interim analysis found that none of the drugs had an effect on in-hospital mortality, the primary outcome, or on initiation of ventilation or duration of hospital stay. There was no standard for the timing of administration of the study drugs in relation to the onset of symptoms.4
Clinical trials of a nebulized formulation of remdesivir for treatment of COVID-19 in outpatients are ongoing.5 A clinical trial (CARAVAN) evaluating IV remdesivir for treatment of COVID-19 in children <18 years old is underway.
TIMING OF ADMINISTRATION — In other viral infections (e.g., influenza), early use of an effective antiviral drug has been associated with improved clinical outcomes. In most patients with COVID-19, replication-competent virus has not been recovered after 10 days following symptom onset. Recovery of replication-competent virus between 10 and 20 days after symptom onset has been documented in some patients with severe COVID-19.6
In ACTT-1, the median duration between symptom onset and randomization was 9 days; the benefit of remdesivir was larger when given earlier in the illness. In SIMPLE-Moderate, the median duration of symptoms before remdesivir treatment was 9 days in the standard treatment group and 8 days in both remdesivir groups. In SIMPLE-Severe, it was 8 days in the 5-day group and 9 days in the 10-day group.
ADVERSE EFFECTS — The most common adverse effects (frequency ≥5%) of remdesivir in the clinical trials were nausea and transaminase elevations. Hypersensitivity reactions, including anaphylaxis, have been reported.
PREGNANCY AND LACTATION — Pregnant women with COVID-19 are at risk for serious morbidity and mortality. In animal studies, no adverse effects on embryofetal development were observed when remdesivir was given to pregnant animals at exposures 4 times the exposure in humans at the recommended dose. In an uncontrolled study in 86 pregnant women with severe COVID-19, remdesivir appeared to be effective (reduced oxygen requirement, high rate of recovery) and safe for use during pregnancy.7 No data are available on the presence of remdesivir in human breast milk or on its effects on the breastfed infant or milk production.
DRUG INTERACTIONS — Coadministration of remdesivir with chloroquine or hydroxychloroquine is not recommended based on cell culture data showing an antagonistic effect of these drugs on the intracellular metabolic activation and antiviral activity of remdesivir. No drug-drug interaction studies of remdesivir have been conducted in humans.
DOSAGE, ADMINISTRATION, AND COST — The recommended dosage of remdesivir is 200 mg IV on day 1, followed by 100 mg once daily. The drug should be infused over 30-120 minutes. The recommended treatment duration is 10 days for patients who need mechanical ventilation and/or extracorporeal membrane oxygenation (ECMO) and 5 days for those who do not. Patients given a 5-day course of remdesivir who do not improve can receive up to 5 additional days of treatment. The drug is not recommended for use in patients with an eGFR <30 mL/min. A 5-day treatment course with Veklury costs $3120.8
CONCLUSION — Clinical trials of the IV antiviral drug remdesivir (Veklury), the first drug to be approved by the FDA for treatment of COVID-19, have produced mixed results. The drug has been shown to shorten the time to recovery in hospitalized adults. The benefit of remdesivir appears to be greater when given earlier in the illness.
- JH Beigel et al. Remdesivir for the treatment of Covid-19 – final report. N Engl J Med 2020; 383:1813.
- CD Spinner et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID 19: a randomized clinical trial. JAMA 2020; 324:1048.
- JD Goldman et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020; 383:1827.
- WHO Solidarity trial consortium. Repurposed antiviral drugs for COVID-19 – interim WHO SOLIDARITY trial results. MedRxiv 2020 October 15 (preprint). Available at: https://bit.ly/3f6OJdf. Accessed November 19, 2020.
- Treatments considered for COVID-19. Med Lett Drugs Ther 2020; 62:e1.
- CDC. Duration of isolation and precautions for adults with COVID-19. October 19, 2020. Available at: https://bit.ly/3pjRtZs. Accessed November 19, 2020.
- RM Burwick et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis 2020 October 8 (epub).
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. November 5, 2020. Reprinted with permission by First Databank, Inc. All rights reserved. ©2020. www.fdbhealth.com/drug-pricing-policy.