ISSUE1631
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of casirivimab and imdevimab used together (REGEN-COV) for post-exposure prophylaxis of COVID-19.
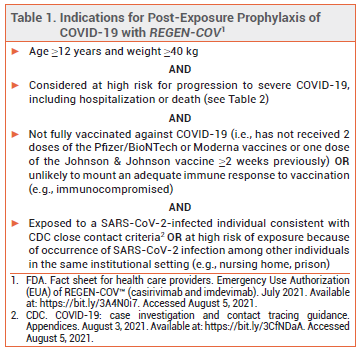
The investigational monoclonal antibodies casirivimab and imdevimab (REGEN-COV – Regeneron) have been available in the US under an Emergency Use Authorization (EUA) since late 2020 for use together to treat mild to moderate COVID-19 in persons ≥12 years old who weigh ≥40 kg and are at high risk of progression to severe disease or hospitalization.1 The FDA has now expanded this EUA to allow use of the antibodies together for post-exposure prophylaxis of COVID-19 in such persons, if they are not fully vaccinated against COVID-19 or are unlikely to have an adequate immune response to full vaccination and have been in close contact with a SARS-CoV-2-infected individual or are likely to be exposed to SARS-CoV-2 in the setting of an institutional outbreak (see Table 1).2 Casirivimab and imdevimab are the first drugs to receive an EUA for post-exposure prophylaxis of COVID-19.
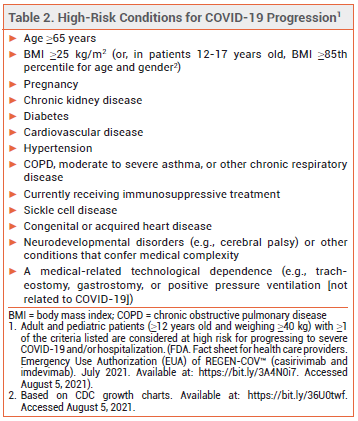
ELIGIBILITY — In May 2021, the FDA expanded the criteria by which a patient with COVID-19 can be considered at high risk for disease progression. All persons ≥12 years old who are overweight or pregnant or have cardiovascular disease, hypertension, or chronic respiratory disease are now considered high-risk (see Table 2).3
CLINICAL STUDIES — Expansion of the EUA was based on the results of a randomized, double-blind, placebo-controlled trial in 1505 healthy, unvaccinated patients ≥12 years old without evidence of prior immunity who were household contacts of persons with SARS-CoV-2 infection (positive test within the prior 96 hours). Patients received a single subcutaneous dose of casirivimab and imdevimab (600 mg each) or placebo.
Symptomatic SARS-CoV-2 infection within 4 weeks of randomization, the primary endpoint, occurred significantly less often in patients who received the antibodies than in those who received placebo (1.5% vs 7.8%; adjusted OR 0.17 [95% CI 0.09-0.33]; NNT 15.4). Among patients who developed symptomatic infection, the duration of symptoms was significantly shorter in the antibody group (mean 1.2 vs 3.2 weeks with placebo). There were no hospitalizations or emergency department visits due to COVID-19 in the antibody group, compared to 4 in the placebo group.4
VARIANTS — Casirivimab plus imdevimab is not active against the Omicron variant of SARS-CoV-2. The combination retains activity against the Delta variant of the virus.2
ADVERSE EFFECTS — Infusion- and injection-related reactions and anaphylaxis have been reported with use of casirivimab and imdevimab.
DOSAGE AND ADMINISTRATION — The authorized dosage of REGEN-COV for post-exposure prophylaxis is 600 mg of casirivimab and 600 mg of imdevimab given as either 4 consecutive SC injections at one time or a single IV infusion. There is no preference for IV over SC administration of REGEN-COV when it is used for post-exposure prophylaxis. In patients with ongoing exposure to SARS-CoV-2, additional 300-mg doses of casirivimab and imdevimab can be administered every 4 weeks. Detailed instructions on preparation and administration of the antibodies are available in the FDA Fact Sheet.2
CONCLUSION — The FDA has authorized the monoclonal antibodies casirivimab and imdevimab (REGEN-COV) to be administered together for post-exposure prophylaxis of COVID-19 in certain high-risk individuals. In a double-blind trial in household contacts of SARS-CoV-2-infected persons, subcutaneous administration of REGEN-COV reduced the risk of symptomatic infection significantly more than placebo. Casirivimab plus imdevimab has retained activity against all SARS-CoV-2 Variants of Concern to date.
- An EUA for casirivimab and imdevimab for COVID-19. Med Lett Drugs Ther 2020; 62:201.
- FDA. Fact sheet for health care providers. Emergency Use Authorization (EUA) of REGEN-COV™ (casirivimab and imdevimab). December 2021. Available at: https://bit.ly/3A4N0i7. Accessed January 6, 2022.
- FDA News Release. Coronavirus (COVID-19) update: May 21, 2021. Available at: https://bit.ly/3fFoEUB. Accessed August 5, 2021.
- MP O'Brien et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med 2021 August 4 (epub).