ISSUE1610
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
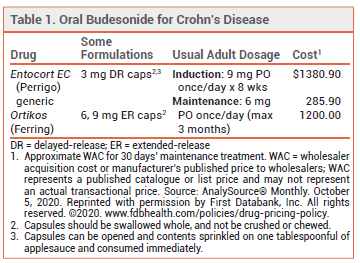
- Compare the new oral, extended-release formulation of budesonide (Ortikos) with other available oral budesonide formulations for treatment of Crohns disease.
An oral extended-release formulation of the corticosteroid budesonide (Ortikos – Ferring) is now available for once-daily treatment of mild to moderate active Crohn’s disease of the ileum and/or ascending colon in patients ≥8 years old and for maintenance of remission for up to 3 months in adults. Ortikos is the second oral formulation of budesonide to be approved for this indication; Entocort EC, an ileal-release formulation, was the first.1 A third oral formulation of budesonide (Uceris) is approved for induction of remission in patients with mild to moderate active ulcerative colitis.

Corticosteroids are effective for short-term symptom control and induction of remission in mild to moderate Crohn's disease. Budesonide is a synthetic corticosteroid with a strong affinity for glucocorticoid receptors and a high ratio of local anti-inflammatory to systemic effects.2
No new clinical trials were required for approval of Ortikos; approval was based on the results of earlier trials with Entocort EC in patients with mild to moderate Crohn's disease.
Oral budesonide is a substrate of CYP3A4; coadministration of CYP3A4 inhibitors including grapefruit juice could increase budesonide serum concentrations and possibly its toxicity.3
The only apparent advantage of Ortikos over Entocort EC is that patients can take one capsule rather than 2 or 3 capsules once daily.
- Drugs for inflammatory bowel disease. Med Lett Drugs Ther 2018; 60:107.
- GR Lichtenstein et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018; 113:481.
- Inhibitors and inducers of CYP enzymes and P-glycoprotein. Med Lett Drugs Ther 2020 September 10 (epub). Available at: medicalletter.org/downloads/CYP_PGP_Tables.pdf.
