ISSUE1620
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of the Johnson & Johnson COVID-19 vaccine.
- Clinical Studies
- Adverse Effects
- Pregnancy and Lactation
- Administration and Storage
- Immunization Priority
- References
Table
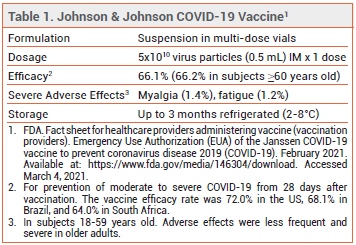
On February 27, 2021, the FDA issued an Emergency Use Authorization (EUA) for the Johnson & Johnson adenovirus-based vaccine for prevention of COVID-19 in persons ≥18 years old. It is the third COVID-19 vaccine to become available in the US and the first to require only a single dose. Two-dose mRNA-based vaccines manufactured by Pfizer-BioNTech and Moderna received EUAs in December 2020.1,2
CLINICAL STUDIES — Issuance of the EUA was based on the results of a double-blind trial (ENSEMBLE; summarized in the FDA fact sheet and briefing document) in which 44,325 adults were randomized to receive a single dose of the Johnson & Johnson vaccine or placebo. The overall vaccine efficacy rate for prevention of moderate to severe COVID-19 from day 28 was 66.1%; it was 72.0% in the US, 68.1% in Brazil, where a SARS-CoV-2 variant of the P.2 lineage caused 69.3% of all sequenced cases, and 64.0% in South Africa, where a SARS-CoV-2 variant from the B.1.351 lineage caused 94.5% of all sequenced cases. In adults ≥60 years old, the vaccine efficacy rate was 66.2%.
For prevention of severe COVID-19 from day 28, the overall vaccine efficacy rate was 85.4%; it was 85.9% in the US, 87.6% in Brazil, and 81.7% in South Africa. Hospitalization due to COVID-19 occurred significantly less often in vaccinated persons than in those who received placebo (2 vs 29 cases ≥14 days after injection; 0 vs 16 cases ≥28 days after injection). No COVID-19-related deaths occurred in the vaccine group, compared to 7 in the placebo group (all in South Africa).3,4
A trial evaluating a two-dose regimen of the Johnson & Johnson vaccine (ENSEMBLE 2) is ongoing.5
ADVERSE EFFECTS — Headache, fatigue, myalgia, nausea, fever, and injection-site pain, erythema, and swelling were reported with the Johnson & Johnson vaccine in ENSEMBLE. Adverse effects were more frequent and severe in persons 18-59 years old than in older adults. Serious adverse events were more common in the placebo group than in the vaccine group.3
Severe hypersensitivity reactions, including anaphylaxis, have been reported with use of the Johnson & Johnson vaccine.4 The product is contraindicated for use in persons with a history of severe allergic reaction to any of its components, including polysorbate.6 The CDC has issued guidance on management of anaphylaxis risk related to COVID-19 vaccination.7
PREGNANCY AND LACTATION — Pregnant women with COVID-19 are at increased risk for morbidity and mortality. According to the FDA, data on the Johnson & Johnson vaccine are insufficient to inform vaccine-associated risk in pregnancy. No data on the effects of the vaccine on the breastfed infant or on milk production are available.3 The American College of Obstetricians and Gynecologists (ACOG) recommends that the vaccine not be withheld from pregnant or lactating women who are otherwise eligible for vaccination.8
ADMINISTRATION AND STORAGE — The Johnson & Johnson vaccine is supplied in cartons of 10 vials, each containing five 0.5-mL doses. It is administered as a single dose by IM injection. The vaccine is initially stored frozen by the manufacturer but shipped refrigerated at 2-8 °C; vaccine that is still frozen upon delivery should be allowed to thaw in the refrigerator or, if necessary, can be thawed at room temperature for 1 hour (if thawing a single vial) or 2 hours (if thawing a carton). Once thawed, the vaccine should not be refrozen.3
Unpunctured vials can be stored at 2-8°C for up to 3 months or at 9-25°C for up to 12 hours. After the first dose has been withdrawn, vials can be stored at 2-8°C for up to 6 hours or at 9-25°C for up to 2 hours. Johnson & Johnson plans to deliver 100 million doses of the vaccine to the US during the first half of 2021.3,9
IMMUNIZATION PRIORITY — The CDC Advisory Committee on Immunization Practices (ACIP) recommends that healthcare personnel and long-term care facility residents be immunized first. Frontline essential workers and adults ≥75 years old are in the second priority group, and persons 65-74 years old, persons 16-64 years old with underlying medical conditions, and other essential workers are in the third.10
The CDC has required state and local jurisdictions to develop vaccination plans for various phases of supply availability. Vaccines will generally be allocated to states and other jurisdictions based on population. State executives and health departments will be responsible for interpreting ACIP guidance and determining where the vaccine should be shipped and who should receive it.11
- FDA authorizes Pfizer-BioNTech COVID-19 vaccine. Med Lett Drugs Ther 2021; 63:1.
- FDA authorizes Moderna COVID-19 vaccine. Med Lett Drugs Ther 2021; 63:9.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). February 2021. Available at: https://bit.ly/3e6KEaD. Accessed March 4, 2021.
- FDA Briefing Document. Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee meeting. February 26, 2021. Available at: https://bit.ly/2PnDJiV. Accessed March 4, 2021.
- NIH. A study of Ad26.COV2.S for the prevention of SARS-CoV-2-mediated COVID-19 in adults (ENSEMBLE 2). Available at: http://bit.ly/3tjvYdm. Accessed March 4, 2021.
- CDC. Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. March 3, 2021. Available at: https://bit.ly/38i7CIH. Accessed March 4, 2021.
- CDC. Interim considerations: preparing for the potential management of anaphylaxis after COVID-19 vaccination. February 10, 2021. Available at: http://bit.ly/3bRK6Tu. Accessed March 4, 2021.
- ACOG. Vaccinating pregnant and lactating patients against COVID-19. February 4, 2021. Available at: http://bit.ly/2Kt7AnS. Accessed March 4, 2021.
- Johnson & Johnson Press Release. Johnson & Johnson COVID-19 vaccine authorized by U.S. FDA for emergency use — first single-shot vaccine in fight against global pandemic. February 27, 2021. Available at: http://bit.ly/384VnhX. Accessed March 4, 2021.
- CDC. CDC’s COVID-19 vaccine rollout recommendations. February 19, 2021. Available at: http://bit.ly/3e3dadm. Accessed March 4, 2021.
- CDC. COVID-19 vaccination program operational guidance. February 16, 2021. Available at: http://bit.ly/2PtMok7. Accessed March 4, 2021.