ISSUE1629
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Discuss the evidence supporting FDA-approval of dapagliflozin (Farxiga) for use in patients with chronic kidney disease.
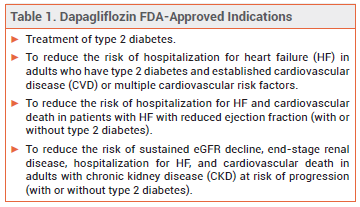
The sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga – AstraZeneca) has been approved by the FDA for treatment of adults with chronic kidney disease (CKD) at risk of progression (not defined in the label). Dapagliflozin is the first SGLT2 inhibitor to be approved in the US for treatment of CKD.
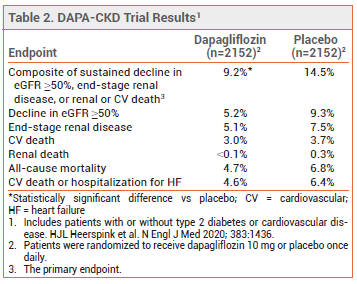
CLINICAL STUDIES – The latest FDA approval was based on the results of a double-blind trial (DAPA-CKD) in 4304 patients with CKD (eGFR 25-75 mL/min/1.73 m2; urine albumin-to-creatinine ratio 200-5000 mg/g) with or without type 2 diabetes or cardiovascular disease. Patients were randomized to receive dapagliflozin 10 mg or placebo once daily in addition to standard treatment. Over a median of 2.4 years, the incidence of the primary endpoint, a composite of sustained decline in eGFR ≥50%, end-stage renal disease, or renal or cardio vascular death, was significantly lower with dapagliflozin than with placebo (see Table 2). The beneficial effects of dapagliflozin were similar in patients with and without type 2 diabetes or cardiovascular disease.1,2
ADVERSE EFFECTS – SGLT2 inhibitors, including dapagliflozin, can increase the risk of genital mycotic infection, urinary tract infection, volume depletion, hypotension, and ketoacidosis in patients with type 2 diabetes. In DAPA-CKD, rates of adverse events were similar in patients with and without type 2 diabetes or cardiovascular disease.
DOSAGE AND COST – The recommended dosage of dapagliflozin for the new indication is 10 mg once daily. The wholesale acquisition cost for 30 days' treatment with Farxiga is $532.80.3
- HJL Heerspink et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383:1436.
- JJV McMurray et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation 2021; 143:438.
- WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. July 5, 2021. Reprinted with permission by First Databank, Inc. All rights reserved. ©2021. www.fdbhealth.com/policies/drug-pricing-policy.