ISSUE1638
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Review the recommendations for use of COVID-19 vaccine booster doses based on level of risk and previous vaccination history.
The FDA has expanded the Emergency Use Authorizations (EUAs) for the mRNA-based COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) and the adenovirus-based vaccine manufactured by Johnson & Johnson/Janssen to include administration of a booster dose in select populations after primary immunization with either the same COVID-19 vaccine or a different one.
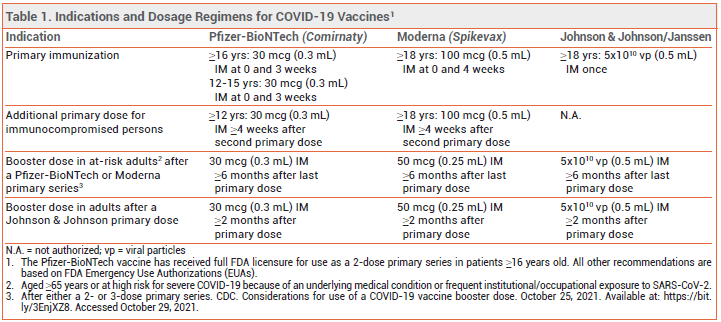
RECOMMENDATIONS — A single booster dose of any COVID-19 vaccine can now be administered ≥6 months after a primary series of an mRNA-based vaccine in adults who are ≥65 years old or at high risk for severe COVID-19 because of an underlying medical condition or frequent institutional or occupational exposure to SARS-CoV-2, or ≥2 months after a single primary dose of the Johnson & Johnson vaccine in any adult (see Table 1).1,2 Revised: As of November 19, 2021, the FDA has expanded the EUAs for the mRNAbased COVID-19 vaccines (Comirnaty and Spikevax) to include administration of a booster dose for all adults ≥18 years old after primary immunization with either the same COVID-19 vaccine or a different one.19
DOSAGE — The booster dose is the same as the dose for primary immunization for both Comirnaty (30 mcg [0.3 mL] IM) and the Johnson & Johnson vaccine (5x1010 viral particles [0.5 mL] IM); it is half the dose for primary immunization for Spikevax (50 mcg [0.25 mL] vs 100 mcg [0.5 mL] IM).3-5
CLINICAL STUDIES — Waning Immunity – In a retrospective cohort study of ~3.4 million persons ≥12 years old in the US, those who received two doses of Comirnaty were significantly less likely to be infected with SARS-CoV-2 than those who were not vaccinated, but the relative risk reduction associated with vaccination declined from 88% at ≤1 month to 47% at ≥5 months after the second dose. Vaccination was also associated with a lower risk of hospitalization due to COVID-19; the relative risk reduction did not change significantly over time (87% at ≤1 month; 88% at ≥5 months).6
In a study in Israel that examined positive PCR test results for SARS-CoV-2 infection over 3 weeks in July 2021, adults ≥60 years old who completed a 2-dose primary series of the Pfizer/BioNTech vaccine in the second half of January 2021 had a significantly higher rate of infection than those who completed their series in the second half of March 2021 (3.3 vs 1.7 cases/1000 persons). Similarly, adults ≥60 years old who completed their series in January had a significantly higher rate of severe COVID-19 than those who completed it in March (0.34 vs 0.15 cases/1000 persons).7
In a US study of breakthrough infection rates between July 1 and August 27, 2021 in ~26,000 adults who had received primary immunization with Spikevax, the rate in an earlier-vaccinated (median 13 months since first dose) cohort was 36.4% higher than that in a later-vaccinated (median 8 months since first dose) cohort (77.1 vs 49.0 cases/1000 person-years).8
The efficacy of the Johnson & Johnson vaccine appears to be sustained through at least 6 months post-dose, but the peak efficacy of a single Johnson & Johnson dose seems to be lower than that of a 2-dose primary series of an mRNA-based vaccine.9-11
Booster Immunogenicity – Longitudinal immunogenicity studies (unpublished; summarized in FDA Fact Sheets) compared titer levels of anti-SARS-CoV- 2 neutralizing antibodies after a booster dose to those achieved after completion of primary immunization in adults with no evidence of prior SARS-CoV-2 infection. In 210 adults 18-55 years old who received a booster dose of Comirnaty about 6 months after completion of a 2-dose primary series, the geometric mean titer (GMT) 1 month after the booster dose was 3.29-fold higher than it was 1 month after the second primary-series dose.3 In 149 adults who received a 50-mcg booster dose of Spikevax ≥6 months after completion of a 2-dose primary series, the GMT 4 weeks after a 50-mcg booster dose was 1.8-fold higher than it was 4 weeks after the second primary-series dose.5 In 38 adults who received a booster dose of the Johnson & Johnson vaccine 12 weeks after a primary dose, the GMT 4 weeks after the booster dose was 1.6-fold higher than it was 4 weeks after the primary dose.4
Booster Efficacy – In a one-month cohort study in ~1.1 million Israeli residents who had completed a 2-dose primary series of the Pfizer/BioNTech vaccine ≥5 months previously, persons who received a booster dose had significantly lower rates of SARS-CoV-2 infection (by 11.3-fold) and severe COVID-19 (by 19.5-fold) beginning 12 days after administration compared to those who did not.12 In a follow-up analysis, relative reductions in the rates of symptomatic and severe COVID-19 associated with booster immunization persisted through ~2 months after the booster dose, and among persons ≥60 years old, the rate of death due to COVID-19 was 14.7-fold lower beginning 12 days after administration in patients who received a booster dose than it was in those who did not receive one.13
In an unpublished double-blind trial (ENSEMBLE 2; summarized in an FDA presentation), 31,300 persons were randomized to receive two doses of the Johnson & Johnson vaccine or placebo 8 weeks apart. After a median follow-up of 36 days, the vaccine efficacy rate for prevention of moderate to severe COVID-19 from 14 days after the second dose, the primary endpoint, was 75% (94% in the US). There were no cases of severe or critical COVID-19 in vaccine recipients versus 8 in the placebo group. This data analysis was performed in June 2021, before the Delta variant of SARS-CoV-2 became predominant in the US.11
Heterologous ("Mix-and-Match") Boosters – In an unpublished nonrandomized trial (summarized in an FDA presentation), 458 adults who had received primary immunization with one of the three FDA-authorized COVID-19 vaccines ≥12 weeks previously were given a booster dose of either the same vaccine or one of the two other vaccines (for Spikevax, 100-mcg rather than 50-mcg booster doses were used). For all vaccine combinations, the GMT of anti-SARS-CoV-2 neutralizing antibodies increased significantly in the 2 weeks after the booster dose.14
ADVERSE EFFECTS — Adverse effects with a third dose of an mRNA-based COVID-19 vaccine appear to be similar to those with the second primary-series dose.15 Lymphadenopathy occurs more frequently with a booster dose. Booster immunization with mRNA-based COVID-19 vaccines has not been associated with increased rates of hypersensitivity reactions, Bell's palsy, or myocarditis/pericarditis compared to primary immunization.16,17
Adverse effects with a booster dose of the Johnson & Johnson vaccine appear to be similar to those with the primary dose. Booster immunization has not been associated with a higher rate of thrombosis with thrombocytopenia syndrome (TTS) compared to primary immunization with the vaccine. In the UK, the rate of TTS with the second dose of the adenovirus-based COVID-19 vaccine manufactured by AstraZeneca (not authorized for use in the US) has been lower than the rate with the first dose.11,18
No vaccine-related serious adverse effects were reported in the heterologous booster trial. Adverse effects with heterologous and homologous booster immunization appear to be similar.14
CONCLUSION — Booster immunization has been associated with decreased rates of SARS-CoV-2 infection and severe COVID-19. The FDA has authorized use of a single booster dose of a COVID-19 vaccine in certain adults who received a primary series with the Pfizer-BioNTech vaccine (Comirnaty) or the Moderna vaccine (Spikevax) ≥6 months previously and in all adults who received a primary dose of the Johnson & Johnson/Janssen vaccine ≥2 months previously. The vaccine used for the booster dose can be different from the one used for primary immunization.
Additional Content Available: COVID-19 Vaccine Dosing Recommendations
- FDA News Release. Coronavirus (COVID-19) update: FDA takes additional actions on the use of a booster dose for COVID-19 vaccines. October 20, 2021. Available at: https://bit.ly/3vJJPLy. Accessed October 29, 2021.
- CDC News Release. CDC expands eligibility for COVID-19 booster shots. October 21, 2021. Available at: https://bit.ly/3CpoXft. Accessed October 29, 2021.
- FDA. Fact sheet for health care providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). October 20, 2021. Available at: https://bit.ly/37fX1NG. Accessed October 29, 2021.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). October 20, 2021. Available at: https://bit.ly/3e6KEaD. Accessed October 29, 2021.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). October 20, 2021. Available at: https://bit.ly/3nosylA. Accessed October 29, 2021.
- SY Tartof et al. Six-month effectiveness of BNT162b2 mRNA COVID-19 vaccine in a large US integrated health system: a retrospective cohort study. 2021 August 23 (preprint). Available at: https://bit.ly/3CJwzJ8. Accessed October 29, 2021.
- Y Goldberg et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021 October 27 (epub).
- LR Baden et al. Covid-19 in the phase 3 trial of mRNA-1273 during the Delta-variant surge. medRxiv 2021 September 22 (preprint). Available at: https://bit.ly/3Gk9q2N. Accessed October 29, 2021.
- J Sadoff et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384:2187.
- JM Polinski et al. Effectiveness of the single-dose Ad26.COV2.S COVID vaccine. medRxiv 2021 September 16 (preprint). Available at: https://bit.ly/3EdYPVk. Accessed October 29, 2021.
- R Zhang and T Brennan. FDA review of effectiveness and safety of Janssen COVID-19 vaccine (Ad26.COV2.S) booster dose. Emergency Use Authorization amendment. Vaccines and Related Biological Products Advisory Committee Meeting. October 14-15, 2021. Available at: https://bit.ly/3k6a9eD. Accessed October 29, 2021.
- YM Bar-On et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021; 385:1393.
- YM Bar-On et al. Protection across age groups of BNT162b2 vaccine booster against Covid-19. medRxiv 2021 October 7 (preprint). Available at: https://bit.ly/3jy9h1Q. Accessed October 29, 2021.
- KE Lyke. DMID 21-0012 – heterologous platform boost study: mix and match. Vaccines and Related Biological Products Advisory Committee Meeting. October 14-15, 2021. Available at: https://bit.ly/2Zk5NcB. Accessed October 29, 2021.
- AM Hause et al. Safety monitoring of an additional dose of COVID-19 Vaccine — United States, August 12–September 19, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1379.
- J Lee. FDA review of effectiveness and safety of COMIRNATY (COVID-19 vaccine, mRNA) booster dose. Biologics License Application supplement. Vaccines and Related Biological Products Advisory Committee Meeting. September 17, 2021. Available at: https://bit. ly/2ZhqsgQ. Accessed October 29, 2021.
- T Mongeau. FDA review of effectiveness and safety of Moderna COVID-19 vaccine (mRNA-1273) booster dose. Emergency Use Authorization amendment. Vaccines and Related Biological Products Advisory Committee Meeting. October 14, 2021. Available at: https://bit.ly/3Be97mq. Accessed October 29, 2021.
- UK Medicines & Healthcare products Regulatory Agency. Coronavirus vaccine – weekly summary of Yellow Card reporting. October 21, 2021. Available at: https://bit.ly/3pFVHO1. Accessed October 29, 2021.
- In Brief: Booster doses of mRNA-based COVID-19 vaccines for all adults. Med Lett Drugs Ther 2021; 63:202.