ISSUE1642
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Review the efficacy and safety of nirmatrelvir copackaged with ritonavir (Paxlovid) for treatment of COVID-19.
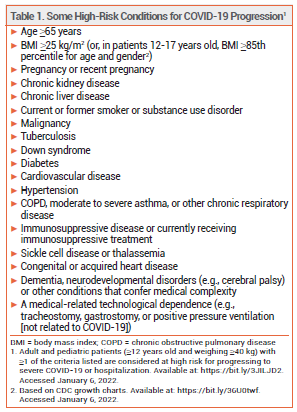
On December 22, 2021, the FDA issued an Emergency Use Authorization (EUA) for the investigational antiviral drug nirmatrelvir copackaged with the HIV-1 protease inhibitor ritonavir (Paxlovid – Pfizer) for oral treatment of mild to moderate COVID-19 in outpatients ≥12 years old who weigh at least 40 kg and are at high risk of progressing to severe disease, including hospitalization or death (see Table 1).1 Paxlovid was the first oral antiviral drug to be authorized in the US for treatment of COVID-19; Merck's oral antiviral drug molnupiravir was granted an EUA for treatment of COVID-19 on December 23, 2021.2 The IV antiviral drug remdesivir (Veklury) was approved by the FDA in 2020 for treatment of COVID-19 in hospitalized patients.3
MECHANISM OF ACTION ― Nirmatrelvir inhibits the SARS-CoV-2 main protease (Mpro), preventing viral replication. Ritonavir does not have any activity against SARS-CoV-2, but it increases serum concentrations of nirmatrelvir by inhibiting its metabolism by CYP3A.
CLINICAL STUDIES ― Issuance of the EUA for nirmatrelvir/ritonavir was based on the results of a randomized, double-blind, placebo-controlled trial (EPIC-HR) in 2246 nonhospitalized, unvaccinated adults with laboratory-confirmed SARS-CoV-2 infection, symptom onset within 5 days of randomization, and at least one risk factor associated with progression to severe disease. Nirmatrelvir 300 mg/ritonavir 100 mg twice daily for 5 days decreased COVID-19 related hospitalization or death through day 28 by 88% compared to placebo (0.8% vs 6.3%). There were 12 deaths in the placebo group versus none in the nirmatrelvir/ritonavir group. Delta was the primary SARS-CoV-2 variant in both groups.1
ADVERSE EFFECTS ― The most common adverse effects of nirmatrelvir/ritonavir in EPIC-HR were dysgeusia, diarrhea, hypertension, and myalgia.
DRUG INTERACTIONS ― Both nirmatrelvir and ritonavir are CYP3A substrates; drugs that induce or inhibit CYP3A will affect serum concentrations of both drugs. Concurrent use of Paxlovid and strong CYP3A inducers, such as rifampin, carbamazepine, phenobarbital, phenytoin, or St. John's wort, can decrease serum concentrations of nirmatrelvir and ritonavir and is contraindicated.4
Ritonavir is a strong inhibitor of CYP3A and may increase serum concentrations of drugs metabolized by CYP3A. Paxlovid is contraindicated for use with drugs that are highly dependent on CYP3A for clearance and for which elevated serum concentrations are associated with serious or life-threatening events (e.g., amiodarone, midazolam). Recommendations for concomitant use of other CYP3A substrates are listed in the FDA Fact Sheet.1 Ritonavir decreases serum concentrations of ethinyl estradiol and may reduce the efficacy of combination hormonal contraceptives.
PREGNANCY AND LACTATION ― There are no data on the use of nirmatrelvir in pregnant women. In animal studies, the drug was associated with reduced fetal weight, but no other adverse effects were detected. Observational data from the antiretroviral pregnancy registry did not show an increase in birth defects following use of ritonavir during pregnancies resulting in more than 6900 live births.
Ritonavir is secreted into human breast milk. No data are available on the presence of nirmatrelvir in human breast milk or the effects of either drug on the breastfed infant or milk production.
VARIANTS ― Nirmatrelvir retains activity against the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) variants. According to the manufacturer, nirmatrelvir also inhibited the 3CL protease associated with the Omicron (B.1.1.529) variant in a biochemical assay.
DOSAGE AND ADMINISTRATION ― Paxlovid is supplied in cartons containing nirmatrelvir 150-mg tablets copackaged with ritonavir 100-mg tablets. The recommended dosage is 300/100 mg (2 nirmatrelvir tablets and 1 ritonavir tablet taken together) twice daily for 5 days. Treatment should be started within 5 days of symptom onset. If a dose is missed by more than 8 hours, it should be skipped and the next dose should be taken at the regularly scheduled time. In patients with moderate renal impairment (eGFR ≥30 to <60 mL/min), the dosage should be reduced to nirmatrelvir 150 mg/ritonavir 100 mg twice daily. Paxlovid is not recommended for use in patients with severe renal impairment (eGFR <30 mL/min) or severe hepatic impairment (Child-Pugh C).
CONCLUSION ― Paxlovid, the investigational oral antiviral drug nirmatrelvir copackaged with oral ritonavir, has received an Emergency Use Authorization from the FDA for treatment of mild to moderate COVID-19 in outpatients ≥12 years old at high risk of progression to severe disease. In one trial, the antiviral combination decreased COVID-19 related hospitalization or death by 88%. It should be started as soon as possible after diagnosis and within 5 days of symptom onset. Paxlovid appears to be well tolerated, but ritonavir is a strong inhibitor of CYP3A and interacts with many other drugs.
- FDA. Fact sheet for health care providers: Emergency Use Authorization for Paxlovid. December 22, 2021. Available at: https://bit.ly/3sTNGqh. Accessed January 6, 2022.
- FDA. Fact sheet for health care providers: Emergency Use Authorization for molnupiravir. December 23, 2021. Available at: https://bit.ly/3FSVSe2. Accessed January 6, 2022.
- Remdesivir (Veklury) for Covid-19. Med Lett Drugs Ther 2020; 62:186.
- Inhibitors and inducers of CYP enzymes, P-glycoprotein, and other transporters. Med Lett Drugs Ther 2021 October 20 (epub). Available at: medicalletter.org/downloads/CYP_PGP_Tables.pdf.