ISSUE1654
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Michael Viscusi, Pharm.D., Associate Editor: no disclosure or potential conflict of interest to report
- Discuss the efficacy and safety of the mRNA COVID-19 vaccines in young children.
The FDA has expanded its Emergency Use Authorizations (EUAs) for the mRNA COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) to allow for their use in children as young as 6 months old. The Pfizer vaccine was previously authorized for use in persons ≥5 years old, and the Moderna vaccine was authorized for use in adults ≥18 years old.1
CLINICAL STUDIES — Expansion of the EUAs of both vaccines was based primarily on immunogenicity data; data on the efficacy of the vaccines in preventing infection and severe disease in younger populations were generally limited by short follow-up times and low infection and hospitalization rates.
A study compared the immunogenicity of three 3-mcg doses of the Pfizer vaccine in 82 children 6-23 months old and 143 children 2-4 years old with that of two 30-mcg doses in 170 persons 16-25 years old. Geometric mean titer levels of anti-SARS-CoV-2 neutralizing antibodies 1 month after the final dose were higher in children 6-23 months old (1406.5) and 2-4 years old (1535.2) than in persons 16-25 years old (1180.0). All children 6 months to 4 years old experienced a seroresponse.2,3
Studies compared the immunogenicity of two doses of the Moderna vaccine in 230 children 6-23 months old (25 mcg), 264 children 2-5 years old (25 mcg), 320 children 6-11 years old (50 mcg), and 340 adolescents 12-17 years old (100 mcg) with that of two 100-mcg doses of the vaccine in cohorts of ~300 adults 18-25 years old. Geometric mean titer levels of anti-SARS-CoV-2 neutralizing antibodies 28 days after the second dose were at least as high in each pediatric cohort as they were in the comparator adult cohorts (1780.7 in children 6-23 months old, 1410.0 in children 2-5 years old, 1610.2 in children 6-11 years old, and 1401.7 in adolescents 12-17 years old vs 1299.9-1390.8 in adults 18-25 years old), and 99-100% of pediatric vaccine recipients experienced a seroresponse.4-6
ADVERSE EFFECTS — In a randomized, observer-blind trial, the most common adverse effects of the Pfizer vaccine in children 6-23 months old were irritability, decreased appetite, fever, and injection-site tenderness, redness, and swelling. Adverse effects in children 2-4 years old included fatigue, fever, headache, chills, muscle pain, and injection-site pain, redness, and swelling. Lymphadenopathy occurred rarely (<0.5%) in both age cohorts. Most adverse effects were mild to moderate in severity. Myocarditis and anaphylaxis due to the vaccine were not reported.2,3
In randomized, observer-blind trials, the most common adverse effects of the Moderna vaccine in children 6-36 months old were irritability, sleepiness, loss of appetite, fever, axillary or groin swelling or tenderness, and injection-site pain, erythema, and swelling. Adverse effects in older children included fatigue, headache, myalgia, arthralgia, fever, chills, nausea/vomiting, axillary or groin swelling or tenderness, and injection-site pain, erythema, and swelling. Most adverse effects were mild to moderate in severity and occurred at a higher frequency after the second dose. Myocarditis and anaphylaxis due to the vaccine were not reported.4-10
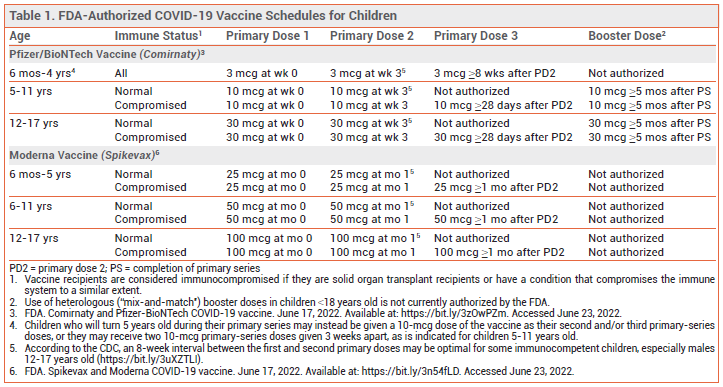
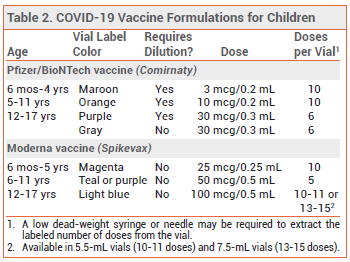
DOSAGE AND ADMINISTRATION — Children receiving the Pfizer vaccine should be given three 3-mcg primary-series doses intramuscularly; the second dose should be given 3 weeks after the first, and the third dose ≥8 weeks after the second. Alternative dosing schedules are authorized for children who will turn 5 years old during their primary series (see Table 1, footnote 4). Booster doses of the Pfizer vaccine are not currently authorized for use in children <5 years old.2
Children receiving the Moderna vaccine should be given two age-appropriate primary-series doses (25 mcg for ages 6 months-5 years, 50 mcg for ages 6-11 years, and 100 mcg for ages 12-17 years) intramuscularly 1 month apart. A third primary-series dose, equal in strength to the first two, should be administered at least 1 month after the second dose to children who have undergone solid organ transplantation or have a condition that compromises the immune system to a similar extent.11 Booster doses of the Moderna vaccine are not currently authorized for use in children <18 years old.4-6
- FDA News Release. Coronavirus (COVID-19) update: FDA authorizes Moderna and Pfizer-BioNTech COVID-19 vaccines for children down to 6 months of age. June 17, 2022. Available at: https://bit.ly/3xObgFQ. Accessed June 23, 2022.
- FDA. Fact sheet for health care providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). For 6 months through 4 years of age. June 17, 2022. Available at: https://bit.ly/3HJhcUT. Accessed June 23, 2022.
- S Wollersheim. FDA review of the effectiveness and safety of Pfizer-BioNTech COVID-19 Vaccine in children 6 months through 4 years of age. Emergency use authorization amendment. Vaccines and Related Biological Products Advisory Committee Meeting. June 15, 2022. Available at: https://bit.ly/3bfM2bs. Accessed June 23, 2022.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Primary series presentation. 6 months through 5 years of age. June 17, 2022. Available at: https://bit.ly/3xJAAMR. Accessed June 23, 2022.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Primary series. 6 years through 11 years of age. June 17, 2022. Available at: https://bit. ly/3tP63vU. Accessed June 23, 2022.
- Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Primary series and booster dose presentation. Primary series doses for 12 years and older. Booster doses for 18 years and older. June 17, 2022. Available at: https://bit.ly/3tP65Uy. Accessed June 23, 2022.
- CB Creech et al. Evaluation of mRNA-1273 Covid-19 vaccine in children 6 to 11 years of age. N Engl J Med 2022; 386:2011.
- K Ali et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med 2021; 385:2241.
- R Wisch. FDA review of effectiveness and safety of Moderna COVID-19 vaccine in children 6 months through 5 years of age. Emergency use authorization amendment. Vaccines and Related Biological Products Advisory Committee Meeting. June 15, 2022. Available at: https://bit.ly/39DnUiE. Accessed June 23, 2022.
- R Zhang. FDA review of effectiveness and safety of Moderna COVID-19 vaccine in children 6 through 17 years of age. Emergency use authorization amendment. Vaccines and Related Biological Products Advisory Committee Meeting. June 15, 2022. Available at: https://bit.ly/3tSxIMO. Accessed June 23, 2022.
- CDC. COVID-19 vaccines for people who are moderately or severely immunocompromised. June 19, 2022. Available at: https://bit.ly/3iREJYo. Accessed June 23, 2022.