ONLY
ARTICLE
The oral kinase inhibitors dabrafenib (Tafinlar – GSK) and trametinib (Mekinist – Novartis) have been approved by the FDA for use together for a sixth indication: treatment of low-grade glioma (LGG) with a BRAF V600E mutation in patients ≥1 years old who require systemic therapy.1,2 This combination is the first systemic therapy to be approved in the US for first-line treatment of LGG with a BRAF V600E mutation in pediatric patients. The FDA also approved new oral formulations of both drugs for patients who are unable to swallow dabrafenib capsules or trametinib tablets.
MECHANISM OF ACTION — BRAF V600E mutations activate the mitogen-activated protein kinase (MAPK) pathway, including BRAF and mitogen-activated extracellular signal regulated kinase 1 (MEK1) and kinase 2 (MEK2), leading to cellular proliferation. Dabrafenib inhibits BRAF V600E and some other mutated forms of BRAF kinases. Trametinib inhibits MEK1 and MEK2 activity. The combination of dabrafenib and trametinib inhibits BRAF V600 mutation-positive tumor cells more than either drug alone.
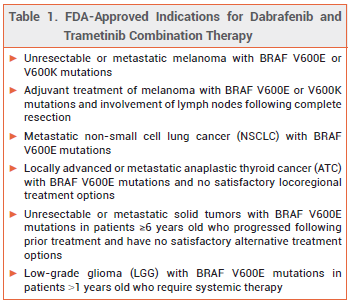
CLINICAL STUDIES — FDA approval of the combination for the new indication was based on the results of an open-label trial (unpublished; summarized in the package insert) in 110 patients 1 to 17 years old with BRAF V600E mutation-positive LGG who required systemic therapy. Patients were randomized to receive dabrafenib plus trametinib or carboplatin plus vincristine. Treatment was continued until loss of clinical benefit or unacceptable toxicity occurred. The overall response rate (ORR) after at least 32 weeks of treatment, the primary endpoint, was statistically significantly higher with dabrafenib/trametinib than with carboplatin/vincristine (46.6% vs 10.8%); most of the responses were partial responses. The duration of response was 23.7 months with dabrafenib/trametinib and not estimable with carboplatin/vincristine. Progression-free survival was also statistically significantly longer with dabrafenib/trametinib (20.1 months vs 7.4 months with carboplatin/vincristine).
ADVERSE EFFECTS – The most common adverse effects of dabrafenib and trametinib combination therapy in pediatric patients were pyrexia (66%), rash (54%), headache (40%), vomiting (38%), musculoskeletal pain (36%), fatigue (31%), dry skin (31%), diarrhea (30%), nausea (26%), epistaxis and other bleeding events (25%), abdominal pain (24%), and dermatitis acneiform (23%). Decreased neutrophil counts and increased alanine aminotransferase and aspartate aminotransferase levels have been reported.
DRUG INTERACTIONS — Dabrafenib is an inducer of UDP-glucuronosyltransferases and multiple CYP isoenzymes, including CYP3A4, 2B6, 2C8, 2C9, and 2C19.3 It may lower serum concentrations of many drugs, including midazolam, warfarin, dexamethasone, and hormonal contraceptives. Dabrafenib is primarily metabolized by CYP3A4 and 2C8; inhibitors or inducers of these enzymes may alter dabrafenib serum concentrations, resulting in loss of efficacy or toxicity.4 Drugs that increase gastric pH, such as proton pump inhibitors, H2-receptor antagonists, or antacids, may reduce the bioavailability of dabrafenib. Trametinib does not affect CYP isoenzymes and has no clinically significant drug interactions.
PREGNANCY AND LACTATION — The combination of dabrafenib/trametinib can cause fetal harm. Both drugs were embryotoxic and teratogenic in animal studies. Females of reproductive potential should be screened for pregnancy before starting dabrafenib/trametinib; they should use an effective nonhormonal contraceptive method while taking the combination and for 4 months after the last dose. Male patients with female partners of reproductive potential should use condoms during treatment with the combination and for at least 4 months after the last dose. No data are available on the effects of the combination on the breastfed infant or milk production. Women should not breastfeed during treatment with these drugs.
DOSAGE AND ADMINISTRATION — Dabrafenib is available in 50- and 75-mg capsules and 10-mg tablets for oral suspension. Trametinib is available in 0.5- and 2-mg tablets and in an oral solution. The recommended doses of dabrafenib and trametinib for pediatric patients are based on body weight; dabrafenib is administered orally twice daily and trametinib is administered orally once daily until disease progression or unacceptable toxicity occurs. Children who weigh 26-37 kg should receive 75 mg of dabrafenib (capsules) twice daily and 1 mg of trametinib (tablets) once daily and those who weigh 38-50 kg should receive 100 mg of dabrafenib (capsules) twice daily and 1.5 mg of trametinib (tablets) once daily.The labels of dabrafenib tablets for oral suspension and trametinib oral solution contain recommended dosages for pediatric patients. Both drugs should be taken 1 hour before or 2 hours after a meal.
- Dabrafenib (Tafinlar) and trametinib (Mekinist) for metastatic melanoma. Med Lett Drugs Ther 2013; 55:62.
- In brief: A new indication for dabrafenib (Tafinlar) and trametinib (Mekinist) combination therapy. Med Lett Drugs Ther 2023; 65:e26.
- H Yin et al. Inhibition of human UDP-glucuronosyltransferase enzyme by dabrafenib: implications for drugdrug interactions. Biomed Chromatogr 2021; 35:e5205. doi:10.1002/bmc.5205
- Inhibitors and inducers of CYP enzymes, P-glycoprotein, and other transporters. Med Lett Drugs Ther 2023 January 25 (epub). Available at: medicalletter.org/downloads/CYP_PGP_Tables.pdf.
