ISSUE1677
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of live fecal microbiota oral capsules (Vowst) for prevention of additional recurrences of Clostridioides difficile infection.
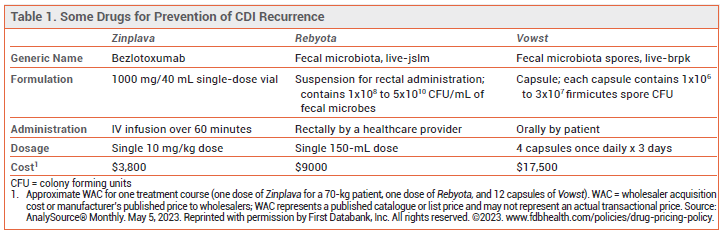
- Description: Oral capsule containing live fecal microbiota spores manufactured from human donor fecal matter.
- Indication: Prevention of additional recurrences of Clostridioides difficile infection (CDI) in adults.
- Efficacy: In one small trial, the rate of CDI recurrence up to 8 weeks after treatment was lower with Vowst than with placebo (12% vs 40%) in patients with ≥3 recurrences within 12 months.
- Adverse Effects: Flatulence, abdominal distension, abdominal pain, fatigue, constipation, chills, and diarrhea can occur.
- Dosage: 4 capsules once daily for 3 days started 2-4 days after the last dose of antibiotics for treatment of CDI.
- Cost: A 3-day course of treatment costs $17,500.
- Conclusion: Vowst reduced the risk of recurrent CDI compared to placebo. Its long-term efficacy remains to be established.
The FDA has approved Vowst (Seres Therapeutics/Nestle HealthScience), an oral capsule containing live fecal microbiota spores, for prevention of additional recurrences of Clostridioides difficile infection (CDI) in adults. Vowst is the first orally administered microbiota-based treatment to be approved for this indication. A rectally-administered live fecal microbiota-based suspension (Rebyota) was approved in 2022 for the same indication.1 Neither product is approved for acute treatment of CDI.

INITIAL EPISODES OF CDI — All adults with an initial episode of nonfulminant CDI should receive 10 days of oral treatment with fidaxomicin (Dificid) or standard-dose vancomycin. Use of fidaxomicin is preferred; compared to vancomycin, it has a narrower spectrum of activity, which limits its effect on the gut microbiome, and it is associated with higher rates of sustained response and fewer recurrences.2,3
RECURRENT CDI — Most CDI recurrences develop within 60 days after stopping antibacterial treatment; the risk is greatest in the first 2 weeks. The recurrence rate after treatment of an initial episode is typically 20-25%. Patients who have one recurrent episode are at increased risk of additional recurrences; the risk of having multiple recurrences has increased in recent years.4
PREVENTION OF RECURRENCES — Addition of a single IV dose of the anti-C. difficile toxin B antibody bezlotoxumab (Zinplava) to antibacterial therapy is recommended to prevent further recurrences in adults who have a recurrence of CDI within 6 months of a previous episode. Use of bezlotoxumab can be considered following antibacterial treatment of a first episode of CDI in patients who have risk factors for disease recurrence (e.g., age ≥65 years, severe CDI, immunosuppression).5 In two trials in patients receiving antibacterial treatment for primary or recurrent CDI, the recurrence rate with addition of bezlotoxumab was significantly lower than that with addition of placebo (16-17% vs 26-28%).6 Bezlotoxumab may exacerbate heart failure.
A single dose of Rebyota (live fecal microbiota rectal suspension) was modestly more effective than placebo in preventing a recurrence of CDI within 8 weeks after treatment of recurrent CDI (estimated treatment success rate of 70.6% vs 57.5%).1
Fecal microbiota transplantation (FMT), a process (not FDA-approved) in which donor feces is administered to the recipient via colonoscopy, upper endoscopy, nasoenteric tube, sigmoidoscopy, an enema, or an oral capsule, has been used for patients with multiple recurrences of CDI.7,8
CLINICAL STUDIES — FDA approval of Vowst was based on the results of a trial (ECOSPOR III) in 182 adults with recurrent CDI (≥3 recurrences within 12 months) who received 10-21 consecutive days of standard antibacterial therapy (vancomycin or fidaxomicin) with improvement in CDI symptoms. Patients were randomized to receive Vowst or placebo for 3 days. Up to 8 weeks after treatment, the rate of CDI recurrence was lower with Vowst than with placebo (12% vs 40%; RR 0.32; number needed to treat [NNT] 3.6).9 The rate of recurrence at 24 weeks, a secondary endpoint, was 21.3% with Vowst and 47.3% with placebo.10
No trials directly comparing Vowst with bezlotoxumab, Rebyota, or FMT for prevention of CDI recurrence are available.
ADVERSE EFFECTS — The most common adverse effects of Vowst in ECOSPOR III were flatulence, abdominal distension, abdominal pain, fatigue, constipation, chills, and diarrhea; most were mild to moderate in severity. Vowst is manufactured from human donor fecal matter that has been tested for a panel of transmissible pathogens, but not for food allergens; the label states that Vowst may transmit infectious agents to the recipient.
DRUG INTERACTIONS — Vowst contains live bacterial spores; concurrent administration of antibacterial drugs is not recommended.
PREGNANCY AND LACTATION — No adequate human or animal data on the use of Vowst during pregnancy are available.
DOSAGE AND ADMINISTRATION — Vowst should be started 2-4 days after completing antibacterial treatment for recurrent CDI and at least 8 hours (preferably 24 hours) after drinking 10 ounces of magnesium citrate (to prevent inactivation of bacterial spores in Vowst by residual fidaxomicin or vancomycin in the GI tract). The recommended dosage is 4 capsules taken once daily before the first meal of the day for 3 consecutive days. The capsules should be swallowed whole.
CONCLUSION — In one small trial, a 3-day course of oral live fecal microbiota spores (Vowst) was more effective than placebo in preventing a recurrence of Clostridioides difficile infection (CDI) within 8 weeks of treatment in patients who had at least 3 recurrences within 12 months. Its long-term efficacy remains to be established. No head-to-head trials comparing Vowst with the anti-C. difficile toxin B antibody bezlotoxumab (Zinplava), a rectally-administered, live fecal microbiota suspension (Rebyota), or fecal microbiota transplant (FMT) are available.
- Live fecal microbiota (Rebyota) for prevention of CDI recurrence. Med Lett Drugs Ther 2023; 65:35.
- Treatment of Clostridioides difficile infection. Med Lett Drugs Ther 2021; 63:137.
- Fidaxomicin (Dificid) for Clostridium difficile infection. Med Lett Drugs Ther 2011; 53:73.
- GK Ma et al. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med 2017; 167:152. doi:10.7326/m16-2733
- S Johnson et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on the management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73:e1029. doi:10.1093/cid/ciab549
- MH Wilcox et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305. doi:10.1056/nejmoa1602615
- CR Kelly et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021; 116;1124. doi:10.14309/ajg.0000000000001278
- NZ Minkoff et al. Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile (Clostridium difficile). Cochrane Database Syst Rev 2023; 4:CD013871. doi:10.1002/14651858.cd013871.pub2
- P Feuerstadt et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med 2022; 386:220. doi:10.1056/nejmoa2106516
- SH Cohen et al. Extended follow-up of microbiome therapeutic SER-109 through 24 weeks for recurrent Clostridioides difficile infection in a randomized clnical trial. JAMA 2022; 328:2062. doi:10.1001/jama.2022.16476