ISSUE1689
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of the updated 2023-2024 COVID-19 vaccine manufactured by Novavax.
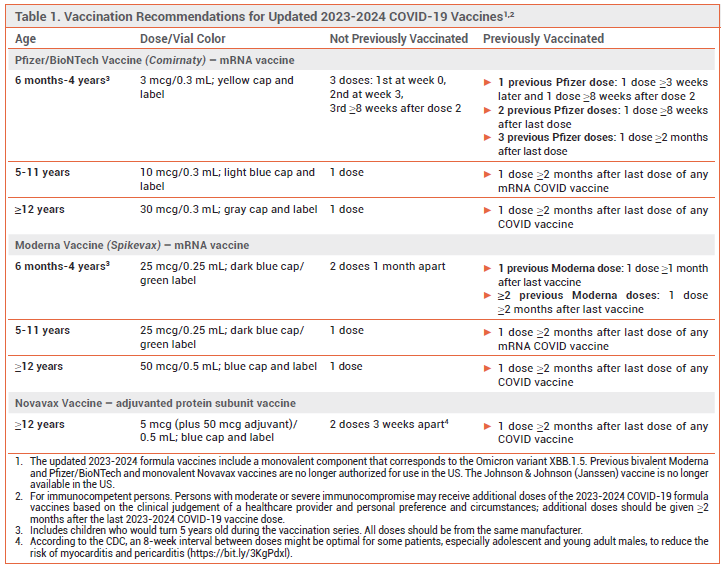
A new 2023-2024 formulation of the adjuvanted protein subunit COVID-19 vaccine manufactured by Novavax that was developed to more closely target currently circulating SARS-CoV-2 variants has been made available in the US under an FDA Emergency Use Authorization (EUA) for use in persons ≥12 years old. The original formulation of the Novavax vaccine is no longer authorized for use in the US.1 In September, updated formulations of the mRNA COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) were licensed by the FDA for use in persons ≥12 years old and made available under EUAs for use in persons 6 months to 11 years old.2
THE NEW FORMULATION – The 2023-2024 formulation of the Novavax vaccine contains the spike protein of the XBB.1.5 Omicron strain of SARS-CoV-2.
EFFICACY – No clinical studies evaluating the effectiveness of the 2023-2024 Novavax vaccine are available. Authorization of the new formulation was based on immunogenicity data showing that it has neutralization potency against currently circulating strains of the virus, including EG.5 (Eris), FL.1.5.1 (Fornax), and various XBB-lineage strains.1,3
ADVERSE EFFECTS – Adverse effects of the original Novavax vaccine have included injection-site pain/tenderness, fatigue/malaise, myalgia, arthralgia, headache, nausea, and vomiting. Hypersensitivity reactions, myocarditis, and pericarditis have occurred rarely with the Novavax and mRNA vaccines; the incidence of myocarditis and pericarditis is highest in adolescent and young adult males.4,5
DOSAGE RECOMMENDATIONS – The updated Novavax COVID-19 vaccine is indicated for use in persons ≥12 years old who have not already received another 2023-2024 COVID-19 vaccine formulation. Generally, persons who have previously been vaccinated against COVID-19 should receive a single 0.5-mL dose ≥2 months after their previous COVID-19 vaccine dose. Those who have not previously been vaccinated against COVID-19 should receive 2 doses; the product labeling recommends that they be given 3 weeks apart, but according to the CDC, an 8-week interval between doses might be optimal for some patients (especially adolescent and young adult males) to reduce the risk of myocarditis and pericarditis. Additional vaccine doses, each given ≥8 weeks after the previous COVID-19 vaccine dose, can be considered for persons with immunocompromise (solid-organ transplant recipients and equivalent).4,5
CDC RECOMMENDATIONS – The CDC recommends that all persons ≥6 months old be immunized with a 2023-2024 COVID-19 vaccine formulation. Persons ≥12 years old can receive a 2023-2024 Pfizer, Moderna, or Novavax vaccine. Persons 6 months to 11 years old should receive a Pfizer or Moderna vaccine.5
- FDA News Release. FDA authorizes updated Novavax COVID-19 vaccine formulated to better protect against currently circulating variants. October 3, 2023. Available at: https://bit.ly/3Fa4Kx8. Accessed October 18, 2023.
- COVID-19 Update: New Pfizer and Moderna vaccine formulations for 2023-2024. Med Lett Drugs Ther 2023; 65:167.
- F Dubovsky. Data in support of Novavax XBB.1.5 vaccine. ACIP. September 12, 2023. Available at: https://bit.ly/48ILUuB. Accessed October 18, 2023.
- FDA. Fact sheet for healthcare providers administering vaccine: Emergency Use Authorization of Novavax vaccine, adjuvanted (2023-2024 formula), for individuals 12 years of age and older. October 3, 2023. Available at: https://bit.ly/46M6kBa. Accessed October 18, 2023.
- CDC. Interim clinical considerations for use of COVID-19 vaccines in the United States. October 6, 2023. Available at: https://bit.ly/3KgPdxl. Accessed October 18, 2023.