Matching articles for "June 28"
In Brief: Only The Name Remains The Same
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
A Medical Letter subscriber was surprised to discover that a new Citracal product contained not only calcium citrate, but also calcium carbonate. Citracal Plus Bone Density Builder actually contains more...
A Medical Letter subscriber was surprised to discover that a new Citracal product contained not only calcium citrate, but also calcium carbonate. Citracal Plus Bone Density Builder actually contains more calcium carbonate per tablet than calcium citrate (240 mg vs. 60 mg). Another Citracal product, Citracal Plus Heart Health, also contains more calcium carbonate than calcium citrate. Many clinicians prefer calcium citrate because it can be taken with or without food, while calcium carbonate must be taken with food for optimal absorption. Other familiar over-the-counter (OTC) names also contain some surprises among their ingredients, as shown in the table below.
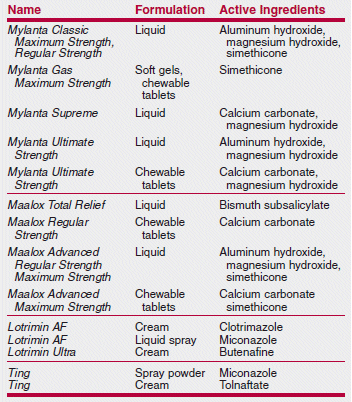
>Many well-known brand-name OTC products no longer contain only or necessarily any of their original ingredients.
Download: U.S. English
Preservation of Ovarian Function During Chemotherapy
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
Chemotherapy can result in premature menopause and infertility in young women. Pretreatment fertility
counseling followed by appropriate action may prevent some of these undesirable...
Chemotherapy can result in premature menopause and infertility in young women. Pretreatment fertility
counseling followed by appropriate action may prevent some of these undesirable consequences.
A Sumatriptan Needle-Free Injector for Migraine
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
Sumatriptan was first marketed in the US in 1993 as Imitrex for subcutaneous injection, followed by tablets for oral administration and then by a nasal spray. It is one of seven serotonin receptor agonists...
Sumatriptan was first marketed in the US in 1993 as Imitrex for subcutaneous injection, followed by tablets for oral administration and then by a nasal spray. It is one of seven serotonin receptor agonists (“triptans”) marketed in the US for treatment of migraine, but it is the only one available for subcutaneous injection. Now the FDA has approved Sumavel DosePro> (Zogenix), a needle-free device for delivering sumatriptan succinate to subcutaneous tissue, for treatment of migraine and cluster headache in adults.
Ofatumumab (Arzerra) for CLL
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
The FDA has approved ofatumumab (Arzerra – GlaxoSmithKline), a human anti-CD20 monoclonal
antibody, for treatment of patients with chronic lymphocytic leukemia (CLL) refractory to fludarabine (Fludara, and...
The FDA has approved ofatumumab (Arzerra – GlaxoSmithKline), a human anti-CD20 monoclonal
antibody, for treatment of patients with chronic lymphocytic leukemia (CLL) refractory to fludarabine (Fludara, and others) and alemtuzumab (Campath). It is the second anti-CD20 antibody approved for treatment of CLL; rituximab (Rituxan), a chimeric murine/human antibody, was the first.
