Matching articles for "Clotrimazole"
Ibrexafungerp (Brexafemme) for Vulvovaginal Candidiasis
The Medical Letter on Drugs and Therapeutics • September 6, 2021; (Issue 1632)
The FDA has approved ibrexafungerp (Brexafemme –
Scynexis), a first-in-class triterpenoid antifungal
("fungerp"), for oral treatment of vulvovaginal candidiasis
in postmenarchal...
The FDA has approved ibrexafungerp (Brexafemme –
Scynexis), a first-in-class triterpenoid antifungal
("fungerp"), for oral treatment of vulvovaginal candidiasis
in postmenarchal females.
Drugs for Sexually Transmitted Infections
The Medical Letter on Drugs and Therapeutics • July 3, 2017; (Issue 1524)
The text and tables that follow include recommendations
for management of sexually transmitted
infections (STIs) other than HIV and viral hepatitis.
Some of the indications and dosages recommended
here have...
The text and tables that follow include recommendations
for management of sexually transmitted
infections (STIs) other than HIV and viral hepatitis.
Some of the indications and dosages recommended
here have not been approved by the FDA.
Luliconazole Cream (Luzu) for Tinea Infections
The Medical Letter on Drugs and Therapeutics • June 23, 2014; (Issue 1445)
The FDA has approved luliconazole (Luzu Cream, 1% –
Valeant) for treatment of tinea pedis, tinea cruris, and
tinea corporis...
The FDA has approved luliconazole (Luzu Cream, 1% –
Valeant) for treatment of tinea pedis, tinea cruris, and
tinea corporis infections.
Drugs for Sexually Transmitted Infections
The Medical Letter on Drugs and Therapeutics • September 1, 2013; (Issue 133)
Many infections can be transmitted during sexual contact.
The text and tables that follow include recommendations
for management of sexually transmitted
infections (STIs) other than HIV, viral hepatitis,...
Many infections can be transmitted during sexual contact.
The text and tables that follow include recommendations
for management of sexually transmitted
infections (STIs) other than HIV, viral hepatitis, and
enteric infections. Some of the indications and
dosages recommended here have not been approved
by the FDA.
Miconazole (Oravig) for Oropharyngeal Candidiasis
The Medical Letter on Drugs and Therapeutics • November 29, 2010; (Issue 1352)
The FDA has approved a buccal tablet formulation of
miconazole (Oravig – Strativa) for local treatment of
oropharyngeal candidiasis in adults. Miconazole has
been available for many years in topical...
The FDA has approved a buccal tablet formulation of
miconazole (Oravig – Strativa) for local treatment of
oropharyngeal candidiasis in adults. Miconazole has
been available for many years in topical formulations
for treatment of superficial fungal infections and vulvovaginal
candidiasis.
Drugs for Sexually Transmitted Infections
The Medical Letter on Drugs and Therapeutics • July 1, 2010; (Issue 95)
Many infections can be transmitted during sexual contact. The text and tables that follow are limited to management of sexually transmitted infections (STIs) other than HIV, viral hepatitis and enteric...
Many infections can be transmitted during sexual contact. The text and tables that follow are limited to management of sexually transmitted infections (STIs) other than HIV, viral hepatitis and enteric infections. The drugs of choice, their dosages and alternatives are listed in a table that begins on page 54. A table listing the adverse effects of some of these antimicrobials begins on page 58.
In Brief: Only The Name Remains The Same
The Medical Letter on Drugs and Therapeutics • June 28, 2010; (Issue 1341)
A Medical Letter subscriber was surprised to discover that a new Citracal product contained not only calcium citrate, but also calcium carbonate. Citracal Plus Bone Density Builder actually contains more...
A Medical Letter subscriber was surprised to discover that a new Citracal product contained not only calcium citrate, but also calcium carbonate. Citracal Plus Bone Density Builder actually contains more calcium carbonate per tablet than calcium citrate (240 mg vs. 60 mg). Another Citracal product, Citracal Plus Heart Health, also contains more calcium carbonate than calcium citrate. Many clinicians prefer calcium citrate because it can be taken with or without food, while calcium carbonate must be taken with food for optimal absorption. Other familiar over-the-counter (OTC) names also contain some surprises among their ingredients, as shown in the table below.
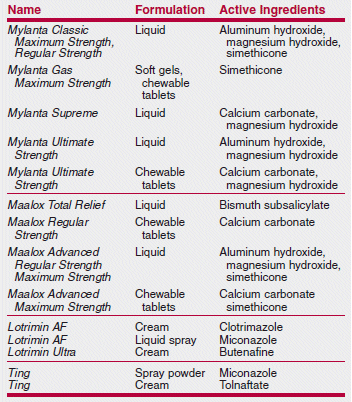
>Many well-known brand-name OTC products no longer contain only or necessarily any of their original ingredients.
Download: U.S. English
Cost of Topical Products for Tinea Pedis
The Medical Letter on Drugs and Therapeutics • May 3, 2010; (Issue 1337)
A Medical Letter reader recently received a diagnosis of athlete’s foot and a prescription for Naftin gel, for which his pharmacy charged $145, and his insurance company required a $70 copay because this...
A Medical Letter reader recently received a diagnosis of athlete’s foot and a prescription for Naftin gel, for which his pharmacy charged $145, and his insurance company required a $70 copay because this formulation was not included in its formulary. Do patients need to pay prices like these to treat tinea pedis?
Antifungal Drugs
The Medical Letter on Drugs and Therapeutics • January 1, 2008; (Issue 65)
The drugs of choice for treatment of some fungal infections are listed in the tables. Some of the indications and dosages recommended here have not been approved by the FDA. Other guidelines are available from...
The drugs of choice for treatment of some fungal infections are listed in the tables. Some of the indications and dosages recommended here have not been approved by the FDA. Other guidelines are available from the Infectious Diseases Society of America (www.idsociety.org).
Drugs for Sexually Transmitted Infections
The Medical Letter on Drugs and Therapeutics • September 1, 2007; (Issue 61)
Many infections can be transmitted during sexual contact. The text and tables that follow are limited to management of sexually transmitted infections (STIs) other than HIV, viral hepatitis and enteric...
Many infections can be transmitted during sexual contact. The text and tables that follow are limited to management of sexually transmitted infections (STIs) other than HIV, viral hepatitis and enteric infections. Guidelines are available from the US Centers for Disease Control and Prevention (CDC) with detailed recommendations for treatment of these diseases.
Drugs for Sexually Transmitted Infections
The Medical Letter on Drugs and Therapeutics • October 1, 2004; (Issue 26)
Many infections can be transmitted during sexual contact. The text and tables that follow are limited to management of sexually transmitted infections (STIs) other than HIV, viral hepatitis and enteric...
Many infections can be transmitted during sexual contact. The text and tables that follow are limited to management of sexually transmitted infections (STIs) other than HIV, viral hepatitis and enteric infections. Guidelines are available from the US Centers for Disease Control and Prevention (CDC) with detailed recommendations for treatment of these diseases (MMWR Recomm Rep 2002; 51, RR-6:1). New guidelines are expected soon.
Topical Sertaconazole (Ertaczo) -- Another Azole for Tinea Pedis
The Medical Letter on Drugs and Therapeutics • June 21, 2004; (Issue 1185)
Sertaconazole nitrate (Ertaczo - OrthoNeutrogena), an imidazole antifungal similar to clotrimazole and miconazole, has been approved by the FDA as a 2% cream for topical treatment of interdigital tinea pedis...
Sertaconazole nitrate (Ertaczo - OrthoNeutrogena), an imidazole antifungal similar to clotrimazole and miconazole, has been approved by the FDA as a 2% cream for topical treatment of interdigital tinea pedis infection. It has been available in Europe for many years.
Generic drugs
The Medical Letter on Drugs and Therapeutics • October 14, 2002; (Issue 1141)
When patents expire on brand-name drugs and generic formulations become available, patients and managed care organizations may express a preference for the lower-cost generics. Are they equivalent to the...
When patents expire on brand-name drugs and generic formulations become available, patients and managed care organizations may express a preference for the lower-cost generics. Are they equivalent to the brand-name product?
Drugs for Sexually Transmitted Infections
The Medical Letter on Drugs and Therapeutics • September 24, 1999; (Issue 1062)
Many infections can be transmitted during sexual contact. The text and tables [in this article] are limited to treatment of non-HIV infections associated primarily with sexual...
Many infections can be transmitted during sexual contact. The text and tables [in this article] are limited to treatment of non-HIV infections associated primarily with sexual transmission.
Topical Penciclovir for Herpes Labialis
The Medical Letter on Drugs and Therapeutics • June 20, 1997; (Issue 1003)
Penciclovir 1% cream (Denavir - SmithKline Beecham) has been approved by the US Food and Drug Administration (FDA) for treatment of recurrent orolabial herpes simplex virus (HSV) infections in adults....
Penciclovir 1% cream (Denavir - SmithKline Beecham) has been approved by the US Food and Drug Administration (FDA) for treatment of recurrent orolabial herpes simplex virus (HSV) infections in adults. Acyclovir (Zovirax) is also available in a topical formulation for treatment of herpes simplex infections, but is approved by the FDA only for use in immunocompromised patients. Oral drugs approved for treatment of some herpes simplex infections, but not recurrent orolabial infections, include acyclovir, valacyclovir (Valtrex) and famciclovir (Famvir), which is rapidly hydrolyzed to penciclovir in vivo (Medical Letter, 36:97, 1994).
Drugs for AIDS and Associated Infections
The Medical Letter on Drugs and Therapeutics • October 13, 1995; (Issue 959)
Results of recently completed clinical trials have led to some changes in recommendation for treatment of human immunodeficiency virus (HIV) and other infections associated with...
Results of recently completed clinical trials have led to some changes in recommendation for treatment of human immunodeficiency virus (HIV) and other infections associated with AIDS.
Oral Fluconazole for Vaginal Candidiasis
The Medical Letter on Drugs and Therapeutics • September 16, 1994; (Issue 931)
Many drugs, mostly imidazole derivatives, are marketed in the USA for topical treatment of vulvovaginal candidiasis (Medical Letter, 33:81, 1991). Recently, fluconazole (Diflucan - Roerig), which is the drug...
Many drugs, mostly imidazole derivatives, are marketed in the USA for topical treatment of vulvovaginal candidiasis (Medical Letter, 33:81, 1991). Recently, fluconazole (Diflucan - Roerig), which is the drug of choice for treatment of oropharyngeal and esophageal candidiasis (Medical Letter, 36:16, 1994), was approved by the US Food and Drug Administration for single-dose oral treatment of .
Drugs for AIDS and Associated infections
The Medical Letter on Drugs and Therapeutics • September 3, 1993; (Issue 904)
Results of recently completed clinical trials have led to some changes in recommendations for treatment of human immunodeficiency virus (HIV) and other infections associted with...
Results of recently completed clinical trials have led to some changes in recommendations for treatment of human immunodeficiency virus (HIV) and other infections associted with AIDS.
Topical Terbinafine for Tinea Infections
The Medical Letter on Drugs and Therapeutics • August 20, 1993; (Issue 903)
Terbinafine 1% cream (Lamisil - Sandoz), an allylamine synthetic antifungal drug chemically related to naftifine (Naftin - Medical Letter, 30:98, 1988), is now available in the USA for topical treatment of...
Terbinafine 1% cream (Lamisil - Sandoz), an allylamine synthetic antifungal drug chemically related to naftifine (Naftin - Medical Letter, 30:98, 1988), is now available in the USA for topical treatment of tinea pedis, tinea cruris, and tinea corporis infections. An oral formulation is available in Europe and is under investigation here.
Itraconazole
The Medical Letter on Drugs and Therapeutics • January 22, 1993; (Issue 888)
Itraconazole (Sporanox - Janssen), an antifungal triazole, has been approved by the US Food and Drug Administration (FDA) for oral treatment of histoplasmosis and blastomycosis. These two endemic mycoses...
Itraconazole (Sporanox - Janssen), an antifungal triazole, has been approved by the US Food and Drug Administration (FDA) for oral treatment of histoplasmosis and blastomycosis. These two endemic mycoses occur both in normal hosts and in immunocompromised patients, such as those with AIDS (RW Bradsher, Clin Infect Dis, 14:S82, 1992; LJ Wheat, Clin Infect Dis, 14:S91, 1992).
Drugs For AIDS And Associated Infections
The Medical Letter on Drugs and Therapeutics • October 18, 1991; (Issue 855)
A growing number of clinical trials now permits some consensus on the treatment of human immunodeficiency virus (HIV) and other infections associated with acquired immune deficiency syndrome (AIDS) in...
A growing number of clinical trials now permits some consensus on the treatment of human immunodeficiency virus (HIV) and other infections associated with acquired immune deficiency syndrome (AIDS) in adults.
Topical Drugs For Vaginal Candidiasis
The Medical Letter on Drugs and Therapeutics • August 23, 1991; (Issue 851)
Many drugs, mostly imidazole derivatives, are marketed in the USA for topical treatment of vulvovaginal candidiasis, and a few are now available without a...
Many drugs, mostly imidazole derivatives, are marketed in the USA for topical treatment of vulvovaginal candidiasis, and a few are now available without a prescription.
Terconazole For Candida Vaginitis
The Medical Letter on Drugs and Therapeutics • December 30, 1988; (Issue 782)
Terconazole (Terazol - Ortho), an imidazole derivative, was recently marketed in the USA for treatment of vulvovaginal candidiasis. It is available both as a 0.4% vaginal cream (Terazol 7) and in 80-mg...
Terconazole (Terazol - Ortho), an imidazole derivative, was recently marketed in the USA for treatment of vulvovaginal candidiasis. It is available both as a 0.4% vaginal cream (Terazol 7) and in 80-mg vaginal suppositories (Terazol 3).
Naftifine For Fungal Skin Infections
The Medical Letter on Drugs and Therapeutics • October 21, 1988; (Issue 777)
Naftifine hydrochloride 1% cream (Naftin - Herbert Laboratories), was recently approved by the US Food and Drug Administration (FDA) for topical treatment of tinea cruris and tinea corporis. It is available...
Naftifine hydrochloride 1% cream (Naftin - Herbert Laboratories), was recently approved by the US Food and Drug Administration (FDA) for topical treatment of tinea cruris and tinea corporis. It is available only by prescription.
