ISSUE1640
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Review the recommendations for use of COVID-19 vaccine booster doses in adults based on previous vaccination history.
Updated: December 9, 2021
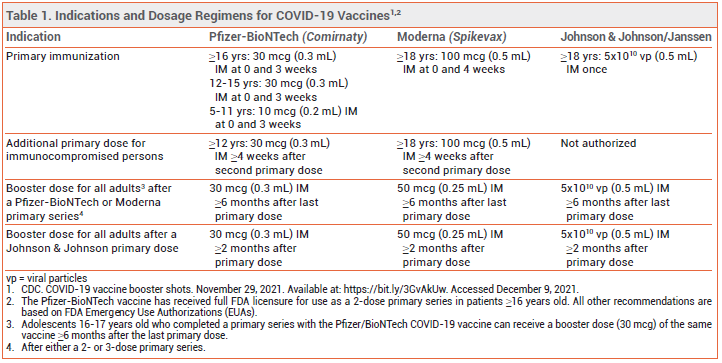
On November 19, the FDA expanded the Emergency Use Authorizations (EUAs) for the mRNA-based COVID-19 vaccines manufactured by Pfizer/BioNTech (Comirnaty) and Moderna (Spikevax) to include administration of a booster dose for all adults ≥18 years old after primary immunization with either the same COVID-19 vaccine or a different one.1 Booster doses of these vaccines were previously authorized only for select populations (age ≥65 years or persons at high risk for severe COVID-19). The EUA for the adenovirus-based vaccine manufactured by Johnson & Johnson was amended in October 2021 to include administration of a booster dose for all adults ≥18 years old after primary immunization with the Johnson & Johnson vaccine.2,3
Note: On December 9, the FDA expanded the EUA of the Pfizer/BioNTech COVID-19 to include a booster dose for adolescents 16-17 years old; the CDC endorsed use of booster doses in this age group. A full review of such use will be published in a future issue.
The CDC recommends a COVID-19 booster dose for all adults ≥18 years old.4 A single booster dose of either mRNA-based COVID-19 vaccine can now be administered ≥6 months after a primary series of an mRNA-based vaccine or ≥2 months after a single primary dose of the Johnson & Johnson vaccine in all adults ≥18 years old.5,6 A single booster dose of the adenovirus-based COVID-19 vaccine can be administered to any adult ≥2 months after a single primary dose of the Johnson & Johnson vaccine or ≥6 months after a primary series of an mRNA-based vaccine (see Table 1).7
Additional Content Available:
COVID-19 Vaccine Dosing Recommendations and Comparison Chart
COVID-19 Vaccine Comparison Chart
- FDA News Release. Coronavirus (COVID-19) update: FDA expands eligibility for COVID-19 vaccine boosters. November 19, 2021. Available at: https://bit.ly/3FQS6S7. Accessed December 9, 2021.
- Booster doses of COVID-19 vaccines. Med Lett Drugs Ther 2021; 63:186.
- FDA News Release. Coronavirus (COVID-19) update: FDA takes additional actions on the use of a booster dose for COVID-19 vaccines. October 20, 2021. Available at: https:/bit.ly/3vJJPLy. Accessed December 9, 2021.
- CDC News Release. CDC expands eligibility for COVID-19 booster shots to all adults. November 19, 2021. Available at: https://bit.ly/3cFk1aJ. Accessed December 9, 2021.
- FDA. Fact sheet for health care providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). November 19, 2021. Available at: https://bit.ly/37fX1NG. Accessed December 9, 2021.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). November 19, 2021. Available at: https://bit.ly/3nosylA. Accessed December 9, 2021.
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency Use Authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). November 19, 2021. Available at: https://bit.ly/3e6KEaD. Accessed December 9, 2021.