ISSUE1401
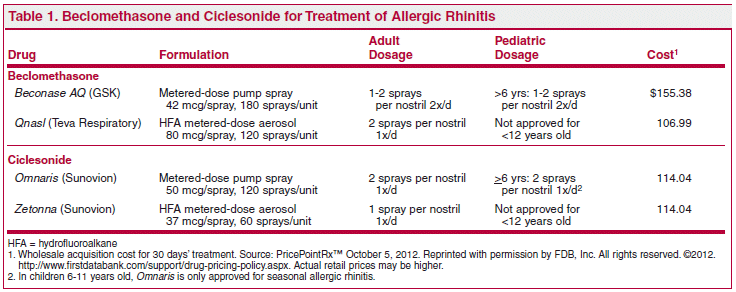
The FDA has approved 2 intranasal HFA (hydrofluoroalkane) aerosols of the corticosteroids beclomethasone dipropionate (Qnasl – Teva Respiratory) and ciclesonide (Zetonna – Sunovion) for once-daily treatment of seasonal and perennial allergic rhinitis. They are the first HFA nasal steroids to become available in the US (HFA propellants do not deplete the ozone layer). Both drugs are already available for these indications as aqueous nasal sprays. Aqueous ("wet") formulations can cause adverse effects such as postnasal drip, moist feeling in the nose, strong odor, and bitter aftertaste, which could reduce patient compliance and may lead to discontinuation of the medication; the new pressurized, odorless, non-aqueous ("dry") HFA formulations may be better tolerated.