ISSUE1674
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Discuss the effect of bempedoic acid (Nexletol) on cardiovascular outcomes in statin-intolerant patients.
Since our initial review of the oral lipid-lowering adenosine triphosphate-citrate lyase (ACL) inhibitor bempedoic acid (Nexletol – Esperion) in 2020,1 cardiovascular outcomes data in statin-intolerant patients have become available.
THE DRUG ― Bempedoic acid is FDA-approved for use alone and in a fixed-dose combination with the cholesterol absorption inhibitor ezetimibe (Nexlizet) as an adjunct to diet and maximally tolerated statin therapy in adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD) who require additional low-density lipoprotein cholesterol (LDL-C) lowering.
Mechanism of Action – The active metabolite of bempedoic acid inhibits ACL, an enzyme involved in hepatic cholesterol synthesis. The resulting reduction in hepatic LDL-C causes upregulation of LDL receptors, increasing clearance of LDL-C from the blood.
STANDARD TREATMENT ― HMG-CoA reductase inhibitors (statins) are the drugs of choice for most patients who require lipid-lowering therapy. They reduce LDL-C levels and the risk of coronary, cerebrovascular, and peripheral vascular events. Some patients may be unwilling or unable to take the guideline-recommended dose of a statin, usually because of myalgia and myopathy.2,3 Reducing the dose or switching to a different statin often allows such patients to continue statin therapy.
Addition of ezetimibe (Zetia, and others) or a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor such as alirocumab (Praluent) or evolocumab (Repatha) to a statin has been shown to further reduce LDL-C levels and the risk of cardiovascular events.
THE CLINICAL TRIAL ― In a double-blind trial (CLEAR Outcomes), 13,970 statin-intolerant patients who had or were at high-risk for cardiovascular disease were randomized to receive bempedoic acid 180 mg or placebo once daily; patients taking a very low dose of a statin or other lipid-lowering drugs (e.g., ezetimibe) were included in the trial.
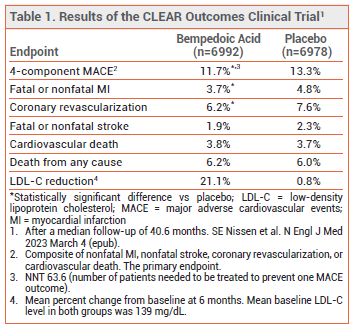
After a median follow-up of 40.6 months, the incidence of a composite of four major adverse cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or cardiovascular death), the primary endpoint, was statistically significantly lower with bempedoic acid than with placebo (11.7% vs 13.3%; number needed to treat [NNT] 63.6). The drug did not significantly reduce the rate of fatal or nonfatal stroke, cardiovascular death, or death from any cause compared to placebo (see Table 1). The mean LDL-C level after 6 months of treatment was lower with bempedoic acid than with placebo (107 mg/dL vs 136 mg/dL), but did not reach goal.4
ADVERSE EFFECTS ― In CLEAR Outcomes, myalgia, new-onset diabetes, worsening hypoglycemia, metabolic acidosis, tendinopathies, and tendon rupture occurred at similar rates with bempedoic acid and placebo.
Bempedoic acid inhibits renal tubular organic anion transporter 2 (OAT2) and may increase serum uric acid levels and the risk of developing gout. In CLEAR Outcomes, gout occurred in 3.1% of patients taking bempedoic acid and in 2.1% of those taking placebo. Cholelithiasis occurred in 3.1% of patients taking bempedoic acid and in 1.2% of those taking placebo.
CONCLUSION ― In one large clinical trial in statinintolerant patients who had or were at high-risk for cardiovascular disease, the oral adenosine triphosphate- citrate lyase (ACL) inhibitor bempedoic acid (Nexletol) modestly reduced the risk of a 4-component composite of major adverse cardiovascular events (MACE) compared to placebo. The drug did not reduce the risk of cardiovascular death or death from any cause. Patients who can tolerate a statin should continue taking it. Whether addition of bempedoic acid to a moderate or maximal dose of a statin further reduces the risk of MACE is unknown.
- Bempedoic acid (Nexletol) for lowering LDL-cholesterol. Med Lett Drugs Ther 2020; 62:53.
- Lipid-lowering drugs. Med Lett Drugs Ther 2022; 64:145.
- JH Alexander. Benefits of bempedoic acid – clearer now. N Engl J Med 2023 March 4 (epub). doi:10.1056/nejme2301490
- SE Nissen et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med 2023 March 4 (epub). doi:10.1056/nejmoa2215024