The Medical Letter on Drugs and Therapeutics
FROM
ISSUE1397
ISSUE1397
Med Lett Drugs Ther. 2012 Aug 20;54(1397):68
Disclosures
Objective(s)
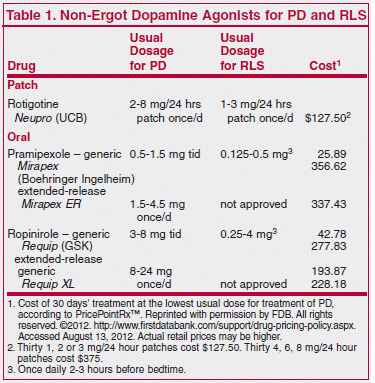
A patch formulation of the non-ergot dopamine agonist rotigotine (Neupro – UCB) has returned to the US market after a 4-year absence. Originally approved by the FDA in 2007 for treatment of early Parkinson’s disease,1 it was withdrawn in 2008 because of crystallization of the drug in the patch, which could have led to under-dosing. The new patch has somewhat broader indications than the old one; it is approved for use in any stage of Parkinson’s disease (PD) and also for moderate-to-severe restless legs syndrome (RLS).
© The Medical Letter, Inc. All Rights Reserved.
The Medical Letter, Inc. does not warrant that all the material in this publication is accurate and
complete in every respect. The Medical Letter, Inc. and its editors shall not be held responsible for any
damage resulting from any error, inaccuracy, or omission.
