ISSUE1522
Opioid use disorder is a chronic, relapsing disease with both physical and psychiatric components. It is associated with economic hardship, social isolation, incarceration, increased rates of blood-borne infections such as HIV and viral hepatitis, adverse pregnancy outcomes, and increased mortality. According to the CDC, there were 33,091 deaths related to opioid overdose in the US in 2015, more than in any previous year.1 Diagnostic criteria for opioid use disorder were updated in the DSM-5.47 Several guidelines on the management of opioid use disorder have recently been published.2-5
MAINTENANCE TREATMENT

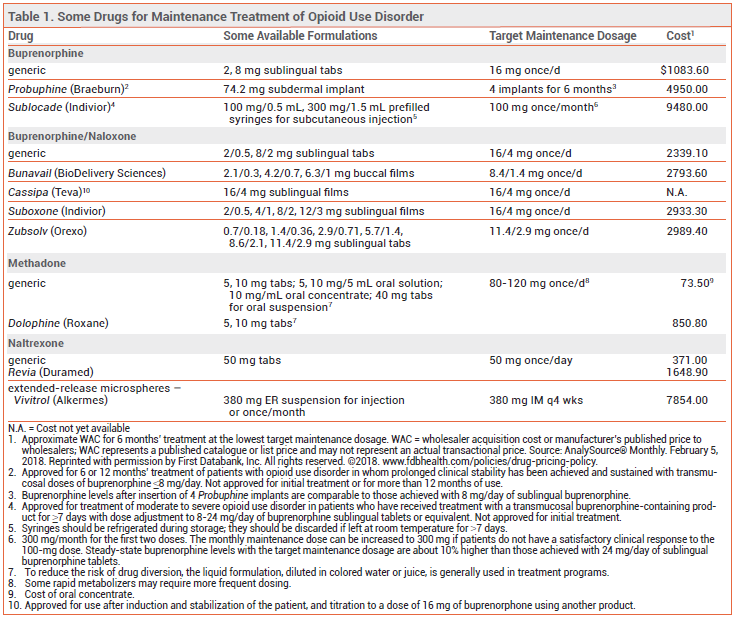
METHADONE — Methadone was the first successful treatment for opioid addiction; it is a synthetic mu-opioid receptor agonist with a slow onset of action and a long, variable elimination half-life. At high doses, methadone induces cross-tolerance with other opioid agonists. Patients tolerant to other opioid agonists, however, may have only an incomplete cross-tolerance to methadone.6
Availability – Methadone is classified as a schedule II controlled substance (highest potential for abuse; recognized medical use). In the US, methadone maintenance treatment is only available through government-licensed opioid treatment programs which offer supervised administration of the drug. The drug is available in oral tablets, tablets for oral suspension, an oral solution, and an oral concentrate. To reduce the risk of drug diversion, treatment programs usually do not dispense the tablet formulation.7
Efficacy – Methadone maintenance therapy can improve treatment retention, productivity, and social engagement, and decrease crime rates, heroin use, injection risk behaviors, mortality rates, and the spread of blood-borne infections such as hepatitis C and HIV.8-11 Use of higher doses of methadone (≥100 mg/day) in patients with opioid use disorder and HIV infection has been associated with increased adherence to antiretroviral therapy, lower viral loads, and higher CD4 cell counts.12
Safety – The risk of methadone-associated mortality is highest in the first weeks after starting or stopping treatment.13 The drug accumulates during induction; it takes 4-7 days to achieve a stable dose. In overdosage, or if the dose is increased too rapidly during initiation of therapy, methadone can cause sedation and respiratory depression. The respiratory depressant effect of methadone peaks later and lasts longer than that of buprenorphine and other opioid agonists, and it persists longer than the analgesic effect of the drug.
Methadone can prolong the QT interval and cause arrhythmias such as torsades de pointes, particularly in patients taking other QT interval-prolonging drugs14 and in those with congenital long QT syndrome or a history of QT-interval prolongation.15
Drug Interactions – Methadone is a substrate of CYP3A4 and CYP2B6; inhibitors of these isozymes can increase serum concentrations of methadone, and inducers can reduce them.16 Concurrent use of methadone and other QT interval-prolonging drugs should be avoided if possible.14 As with any opioid, concomitant use of methadone with selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, or other serotonergic drugs could result in serotonin syndrome. Concurrent use of methadone and benzodiazepines or other sedating drugs can result in additive CNS effects.
Dosage and Administration – Because methadone has a long half-life, initial titration of the drug should be performed slowly. Federal law prohibits administration of an initial methadone dose >30 mg or a total first daily dose >40 mg.17 For most patients, a maintenance dose of 60-120 mg/day can suppress cravings and block the euphoric effects of other opioid agonists.18
For a more detailed table, click here.
BUPRENORPHINE — Buprenorphine is a mu-opioid receptor partial agonist and kappa-opioid receptor antagonist. It is available alone and in combination with the opioid antagonist naloxone.19-22 Taken orally, naloxone is poorly absorbed and generally has no clinical effects; combining it with buprenorphine in sublingual or buccal formulations is intended to counteract intravenous or intranasal abuse.
Availability – Buprenorphine is classified as a schedule III controlled substance (less potential for abuse than schedule II; recognized medical use). In the US, prescribers who complete a training course and obtain a special DEA number can treat a limited number of patients for opioid use disorder with buprenorphine in an outpatient setting; current laws relating to outpatient prescription of buprenorphine are available at the website for the Substance Abuse and Mental Health Services Administration.23
Efficacy – Buprenorphine significantly improves treatment retention and reduces illicit opioid use compared to placebo.24 It appears to be at least as effective as methadone in reducing mortality.13 Office-based buprenorphine/naloxone maintenance therapy has been shown to improve abstinence rates, occupational stability, and psycho social outcomes.25
Administering buprenorphine via subdermal implant (Probuphine)22 or once-monthly extended-release subcutaneous injection (Sublocade)48 should reduce the risks of treatment nonadherence and drug diversion, but these formulations are more expensive than transmucosal buprenorphine. Sublocade has not been studied in active-controlled clinical trials.
Safety – Even without naloxone, buprenorphine is safer than methadone because it has a ceiling on its respiratory depressant effect. As a partial agonist, it also has a lower abuse potential than methadone; the presence of naloxone may further reduce the abuse potential of buprenorphine products.
In a retrospective analysis of about 5 million adverse drug events reported to the FDA over 42 years, events mentioning methadone, but not those mentioning buprenorphine, were disproportionately likely to involve QT-interval prolongaton, ventricular arrhythmia, or cardiac arrest.26
In a retrospective study in the United Kingdom of about 20 million prescriptions for methadone or buprenorphine over a 6-year period, prescriptions for methadone were 6.23 times more likely to be associated with a subsequent overdose death than prescriptions for buprenorphine with or without naloxone.27
Hepatic impairment reduces naloxone clearance to a greater extent than it does buprenorphine clearance. Use of fixed-dose buprenorphine/naloxone combinations in patients with severe hepatic impairment can lead to withdrawal symptoms when treatment is started and may decrease the efficacy of buprenorphine maintenance treatment.
Buprenorphine subdermal implants, like other drug-eluting implants, can cause adverse effects such as pain, pruritus, and erythema at the insertion site, and insertion and removal of implants have been associated with nerve injury and implant migration and extrusion. Healthcare providers must complete a live training session and become certified through a Risk Evaluation and Mitigation Strategy (REMS) program before they can prescribe, insert, or remove buprenorphine implants.22
Injection-site reactions can occur with subcutaneous administration of buprenorphine. Healthcare facilities must become certified through a REMS program before they can order and dispense Sublocade.48
Drug Interactions – Concurrent use of benzodiazepines or other sedating drugs with opioids such as buprenorphine can result in additive effects. Buprenorphine is metabolized primarily by CYP3A4; concomitant use with a 3A4 inducer can decrease buprenorphine serum concentrations and use with a 3A4 inhibitor can increase them.16 Buprenorphine is a substrate of P-glycoprotein (Pgp); concomitant use with inhibitors of P-gp could increase buprenorphine serum concentrations. Use of an opioid with serotonergic drugs such as selective serotonin reuptake inhibitors (SSRIs) may result in serotonin syndrome, though the risk is lower with buprenorphine than it is with directly serotonergic opioids such as methadone.
Buprenorphine may interfere with the analgesic efficacy of full opioid agonists. It should generally be discontinued 24-36 hours before elective surgery (except Cesarean section; stopping buprenorphine could cause fetal withdrawal).
Dosage and Administration – Buprenorphine has a greater affinity for opioid receptors than full opioid agonists such as heroin and can displace them, causing opioid withdrawal. The risk of withdrawal can be reduced by not starting treatment until the patient is already experiencing mild-to-moderate opioid withdrawal (Clinical Opiate Withdrawal Scale score ~11-12)28 and by using a low initial dose of buprenorphine (2-4 mg of Suboxone, or equivalent). The daily dose should then be uptitrated, usually to at least 8 mg; most patients can readily achieve their target dose within 2-3 days. Higher doses (12-16 mg/day) should be considered if opioid misuse or abuse persists. Data supporting increased efficacy with doses >16 mg/day up to the maximum recommended dose of 24 mg/day are limited.
NALTREXONE — The mu-opioid receptor antagonist naltrexone is available as a once-daily oral tablet (Revia, and generics) and as a once-monthly extended-release (ER) microsphere suspension given by intramuscular (IM) injection (Vivitrol). It is not addictive or readily abused, and tolerance to its effects does not develop with long-term use. Both oral and extended-release IM naltrexone are also approved for treatment of alcohol use disorder.29
Availability – Naltrexone is not a controlled substance, and there are no special restrictions on its prescription.
Efficacy – Adherence and outcomes have been better with extended-release IM naltrexone than with the oral formulation, though neither formulation has been conclusively shown to have a mortality benefit. In a randomized, double-blind, placebo-controlled, 24-week trial in 250 patients with opioid dependence, addition of injectable naltrexone to biweekly counseling was found to significantly increase the likelihood of abstinence from opioid use, improve retention in the treatment program, and reduce cravings and relapse to physiological opioid dependence, compared to addition of placebo.30 In an open-label, randomized, 24-week trial in 308 patients with a history of opioid dependence who were abstinent from opioids at the time of randomization, those who received extended-release IM naltrexone had a significantly longer median time to relapse (10.5 vs 5.0 weeks) and significantly lower rates of relapse (43% vs 64%) than those who received usual care, which consisted of brief counseling and referral to community treatment programs.31 In an open-label study, treatment failure during induction occurred significantly more often with once-monthly IM naltrexone than with sublingual buprenorphine/naloxone, but among those successfully inducted, the two treatments were similar in efficacy and safety.49
In a meta-analysis of 13 studies, oral naltrexone was no more effective than placebo, nonpharmacologic treatment, or benzodiazepines or buprenorphine in reducing opioid use, and treatment retention was poor.32 Oral naltrexone was more effective than placebo in sustaining abstinence in studies where patients were legally mandated to take the drug.31-33
Safety – Naltrexone is generally well tolerated. Adverse effects reported in opioid-dependent patients given IM naltrexone have included injection-site reactions, nasopharyngitis, insomnia, headache, nausea, and toothache. Depressed mood and suicidality have occurred rarely; a cause-and-effect relationship has not been established. Hepatic enzyme elevations and toxicity have been reported with use of naltrexone, but these findings occur frequently in opioid- and alcohol-dependent patients. Use of naltrexone can reduce tolerance to opioids; patients who relapse after receiving naltrexone may be at greater risk of a serious, potentially fatal opioid overdose.
Drug Interactions – Naltrexone blocks the effects of usual doses of opioids, including opioid-derivative antidiarrheals and antitussives.34 It should not be used in patients taking an opioid for treatment of pain. Oral naltrexone should be discontinued 72 hours before and IM naltrexone 30 days before elective surgery.
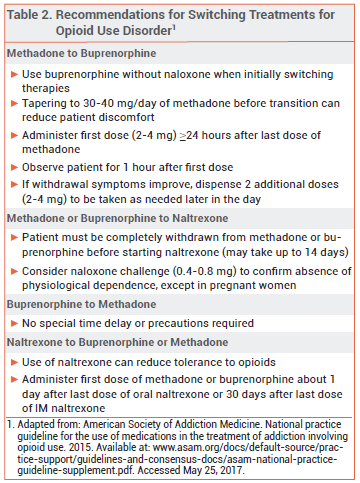
Dosage and Administration – Administration of naltrexone to a patient with physiological opioid dependence can precipitate a severe opioid withdrawal syndrome; patients should be free of dependence for at least 7 days before naltrexone is initiated. A naloxone challenge can be used to confirm the absence of physiological opioid dependence, but it is contraindicated in pregnant women because it can induce preterm labor or fetal distress.
Vivitrol is supplied in single-use cartons containing a vial of microspheres, a diluent for suspension, needles, and a syringe. It should be given as a 380-mg IM gluteal injection in alternating buttocks every 4 weeks or once monthly. The dose pack should be stored in the refrigerator; if left unrefrigerated, it may be used for up to 7 days as long as it is not exposed to temperatures >77°F (25°C).
Patients starting treatment with oral naltrexone should receive an initial dose of 25 mg. If withdrawal symptoms do not occur, they can then be given a maintenance dosage of 50 mg once daily.
ALTERNATIVES — Limited data suggest that 24-hour extended-release oral morphine may be effective for maintenance treatment of opioid use disorder. Morphine may be better tolerated and more effective than methadone in some patients; it has less of an effect on the QT interval, but it may have a greater risk of opioid-related adverse effects.35 In a 22-week, randomized, open-label, crossover study in 157 patients being treated in methadone maintenance clinics, 24-hour oral morphine was noninferior to methadone in preventing positive urine tests for heroin. Adverse effects were similar in the two groups.36
Addition of supervised heroin injections to flexible-dose methadone therapy has been shown to improve treatment retention and may also reduce criminal activity, incarceration rates, and social functioning, but it also increases the risk of adverse events.37
In a 12-week, randomized, double-blind trial in 196 opioid-dependent patients, addition of the antitussive dextromethorphan 60 mg/day to methadone maintenance therapy significantly improved treatment retention and decreased plasma morphine levels compared to placebo, but addition of dextromethorphan 120 mg/day did not.38
In a 24-week randomized trial in 141 opioid-dependent patients, addition of cognitive behavioral therapy to primary care-based maintenance treatment with buprenorphine did not improve self-reported opioid use or opioid abstinence.39
PREGNANCY — Opioid use during pregnancy is associated with an increased risk of complications such as preeclampsia, miscarriage, reduced fetal growth, fetal death, and premature delivery.40 Pregnant women who are physically dependent on opioids or are likely to resume opioid misuse should receive opioid agonist maintenance therapy; it is safer than detoxification alone.41
Methadone has a long history of use in pregnancy and is generally considered the standard of care for maintenance treatment of pregnant women with opioid use disorder. More recently, buprenorphine (without naloxone) has been used as an effective and safe alternative. In a randomized, double-blind, double-dummy trial in 175 opioid-dependent pregnant women, neonates whose mothers were treated with buprenorphine during pregnancy required less morphine and had shorter durations of treatment for neonatal abstinence syn drome and hospital stays than those whose mothers received methadone, but treatment retention was significantly greater among women taking methadone.42
Combination buprenorphine/naloxone products are considered safe for use during pregnancy, but data on their efficacy in pregnant women are limited.43 Buprenorphine without naloxone is preferred.
Data on the safety and efficacy of naltrexone use in pregnancy are limited. In general, women taking naltrexone who become pregnant and are at high risk for relapse can continue treatment.
LACTATION — Use of methadone or buprenorphine monotherapy by breastfeeding women is generally considered safe. Women taking naltrexone should not breastfeed because the drug and its major metabolite can pass into breast milk to a clinically significant extent.
TREATMENT OF OPIOID OVERDOSE
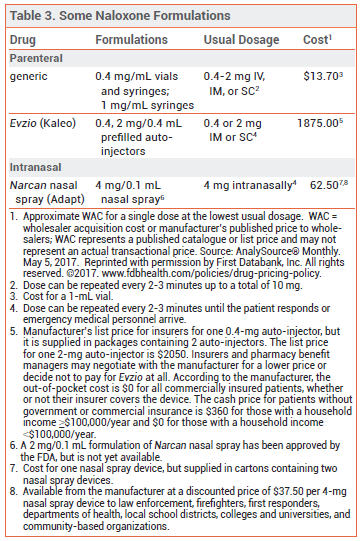
NALOXONE — Naloxone is the drug of choice for emergency treatment of opioid overdose. It is available in various dosage forms for intravenous (IV), intramuscular (IM), subcutaneous (SC), or intranasal administration (see Table 3).44,45
Availability – A number of jurisdictions now have naloxone access laws that make the drug available to first responders and to relatives and close friends of persons using heroin or taking prescription opioids. These laws may also grant civil and criminal immunity to laypeople who carry or administer naloxone, to healthcare professionals who prescribe or dispense the drug to laypeople, and to persons who call emergency medical services in good faith to reverse an overdose. A regularly updated database of state naloxone access laws is available (http://lawatlas.org/datasets/laws-regulating-administration-of-naloxone).
Pharmacology – Naloxone is a competitive mu-opioid receptor antagonist and has no opioid agonist effects. In opioid overdose, naloxone begins to reverse sedation, respiratory depression, and hypotension within 1-2 minutes after IV administration, 2-5 minutes after IM or SC administration, and 8-13 minutes after intranasal administration.
The half-life of naloxone is much shorter than that of most opioids and repeated administration may be necessary, especially for overdose with a long-acting opioid agonist such as methadone or a sustained-release formulation of a short-acting agonist such as oxycodone. Pure heroin has a shorter half-life than naloxone (2-6 minutes), but heroin is a prodrug that is rapidly metabolized to 6-acetylmorphine and morphine. The risk of respiratory depression is related to those active metabolites, and it may persist well beyond the clearance of heroin from the blood. Other drugs used to "cut" heroin may have longer half-lives.46 If not already present, emergency medical services should always be called immediately after administration of naloxone.
Adverse Effects – Whether naloxone itself has any toxicity is unclear, but it can precipitate acute withdrawal symptoms in opioid-dependent patients. Acute opioid withdrawal is associated with anxiety, piloerection, yawning, sneezing, rhinorrhea, nausea, vomiting, diarrhea, and abdominal or muscle cramps, which are uncomfortable but generally not life-threatening, except in neonates. In a pharmacokinetic study of intranasal naloxone (Narcan), the most common adverse effects were increased blood pressure, constipation, toothache, muscle spasms, musculoskeletal pain, headache, rhinalgia, xeroderma, and intranasal effects including dryness, edema, congestion, and inflammation.
Pregnancy – No embryotoxic or teratogenic effects were observed in pregnant mice and rats treated with large doses of naloxone. Naloxone does cross the placenta, however, and may cause fetal opioid withdrawal or induce preterm labor.
View Comparison Table: Some Drugs for Maintenance Treatment of Opioid Use Disorder
- Centers for Disease Control and Prevention. Drug overdose death data. Available at: www.cdc.gov. Accessed May 25, 2017.
- American Society of Addiction Medicine. National practice guideline for the use of medications in the treatment of addiction involving opioid use. 2015. Available at: www. asam.org. Accessed May 25, 2017.
- Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. 2015. Available at: www.healthquality.va.gov. Accessed May 25, 2017.
- MA Schuckit. Treatment of opioid-use disorders. N Engl J Med 2016; 375:357.
- British Columbia Ministry of Health. A guideline for the clinical management of opioid use disorder. Available at: www2.gov.bc.ca. Accessed May 25, 2017.
- Institute for Safe Medication Practices. Keeping patients safe from iatrogenic methadone overdoses. February 14, 2008. Available at: www.ismp.org. Accessed May 25, 2017.
- Substance Abuse and Mental Health Services Administration. Substance abuse treatment advisory. News for the treatment field. Emerging issues in the use of methadone. 2009. Available at: https://store.samhsa.gov. Accessed May 25, 2017.
- RP Mattick et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev 2009; 3:CD002209.
- F Faggiano et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev 2003; 3:CD002208.
- L Gowing et al. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev 2011; 8:CD004145.
- S Nolan et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109:2053.
- L Lappalainen et al. Dose-response relationship between methadone dose and adherence to antiretroviral therapy among HIV-positive people who use illicit opioids. Addiction 2015; 110:1330.
- L Sordo et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357:j1550.
- RL Woosley and KA Romero. QT drugs list. Available at: www. crediblemeds.org. Accessed May 25, 2017.
- FDA. Information for healthcare professionals methadone hydrochloride text version. Available at: www.fda.gov. Accessed May 25, 2017.
- Inhibitors and inducers of CYP enzymes and p-glycoprotein. Med Lett Drugs Ther 2017. May 18 (epub). Available at: medicalletter.org/downloads/CYP_PGP_Tables.pdf. Accessed May 25, 2017.
- U.S. Government Publishing Office. 42 CFR 8.12 – Federal opioid treatment standards. Available at: www.gpo.gov. Accessed May 25, 2017.
- LE Baxter Sr et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med 2013; 7:377.
- Buprenorphine: an alternative to methadone. Med Lett Drugs Ther 2003; 45:13.
- In brief: Buprenorphine/naloxone (Zubsolv) for opioid dependence. Med Lett Drugs Ther 2013; 55:83.
- Bunavail: another buprenorphine/naloxone formulation for opioid dependence. Med Lett Drugs Ther 2015; 57:19.
- Buprenorphine implants (Probuphine) for opioid dependence. Med Lett Drugs Ther 2016; 58:94.
- Substance Abuse and Mental Health Services Administration. Buprenorphine waiver management. 2017. Available at: www. samhsa.gov. Accessed May 25, 2017.
- RP Mattick et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; 2:CD002207.
- TV Parran et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend 2010; 106:56.
- DP Kao et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction 2015; 110:1468.
- D Marteau et al. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open 2015; 5:e007629.
- DR Wesson and W Ling. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003; 35:253.
- Naltrexone (Vivitrol) – a once-monthly injection for alcoholism. Med Lett Drugs Ther 2006; 48:62.
- E Krupitsky et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet 2011; 377:1506.
- JD Lee et al. Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med 2016; 374:1232.
- S Minozzi et al. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 2011; 4:CD001333.
- Y Adi et al. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation. Health Technol Assess 2007; 11:iii.
- SD Comer et al. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology 2002; 159:351.
- M Ferri et al. Slow-release oral morphine as maintenance therapy for opioid dependence. Cochrane Database Syst Rev 2013; 6:CD009879.
- T Beck et al. Maintenance treatment for opioid dependence with slow-release oral morphine: a randomized cross-over, non-inferiority study versus methadone. Addiction 2014; 109:617.
- M Ferri et al. Heroin maintenance for chronic heroin-dependent individuals. Cochrane Database Syst Rev 2011; 12:CD003410.
- SY Lee et al. A placebo-controlled trial of dextromethorphan as an adjunct in opioid-dependent patients undergoing methadone maintenance treatment. Int J Neuropsychopharmacol 2015 February 25 (epub).
- DA Fiellin et al. A randomized trial of cognitive behavioral therapy in primary care-based buprenorphine. Am J Med 2013; 126:74.
- ACOG Committee on Health Care for Underserved Women and American Society of Addiction Medicine. ACOG committee opinion No. 524: opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol 2012; 119:1070.
- KA Saia et al. Caring for pregnant women with opioid use disorder in the USA: expanding and improving treatment. Curr Obstet Gynecol Rep 2016; 5:257.
- HE Jones et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med 2010; 363:2320.
- SL Wiegand et al. Buprenorphine and naloxone compared with methadone treatment in pregnancy. Obstet Gynecol 2015; 125:363.
- In brief: A naloxone auto-injector (Evzio). Med Lett Drugs Ther 2014; 56:45.
- Naloxone (Narcan) nasal spray for opioid overdose. Med Lett Drugs Ther 2016; 58:1.
- EW Boyer. Management of opioid analgesic overdose. N Engl J Med 2012; 367:146.
- American Psychiatric Association. Opioid use disorder: diagnostic criteria. Available at: https://pcssmat.org. Accessed February 15, 2018.
- Once-monthly subcutaneous buprenorphine (Sublocade) for opioid use disorder. Med Lett Drugs Ther 2018; 60:35.
- JD Lee et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 2018; 391:309.