ISSUE1633
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Amy Faucard, MLS, Associate Editor: no disclosure or potential conflict of interest to report
- Compare the pharmacokinetic properties of the new 8-mg intranasal formulation of naloxone (Kloxxado) to other available formulations of the drug.
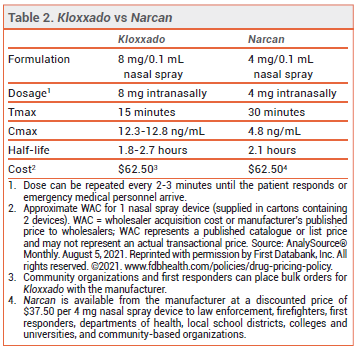
The FDA has approved a higher-dose intranasal naloxone formulation (Kloxxado – Hikma) for emergency treatment of opioid overdose. A single spray of the new formulation delivers 8 mg of naloxone; a formulation that delivers 4 mg per spray (Narcan) was approved in 2015.1

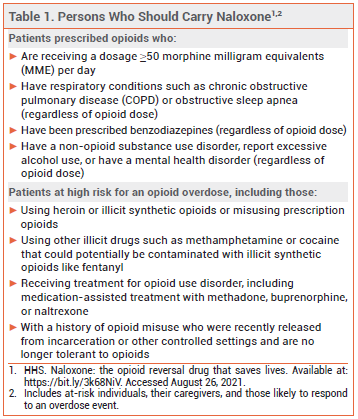
Opioid-related overdose deaths have been increasing in the US and this increase has accelerated during the COVID-19 pandemic.2 Studies of Emergency Medical Services calls for opioid overdose during the pandemic have found an increase in patients receiving multiple doses of naloxone.3 The US Department of Health and Human Services has published recommendations for prescribing or co-prescribing naloxone for individuals who are at risk of opioid overdose (see Table 1).4,5
No clinical data are available on when to use a higher dose of naloxone for reversal of an opioid overdose. A higher dose may be required when the overdose is due to use of illicitly manufactured synthetic opioids such as fentanyl and its analogs, or when it is more severe or has progressed.
CLINICAL STUDIES — No new clinical trials were required for FDA approval of the 8-mg formulation. Two unpublished pharmacokinetic studies (summarized in the package insert) in healthy adults compared a single 8-mg intranasal dose of naloxone to single doses of the drug given IM (0.4 mg) and IV (2 mg). The median time to reach maximum serum concentrations (Tmax) was about 15 minutes with both intranasal and IM administration. The mean maximum naloxone serum concentration following intranasal administration was about 10 times higher than that achieved after IM administration. The IV dose had a faster onset of action than the intranasal dose, but after about 10 minutes, serum concentrations were higher with the nasal spray.
ADVERSE EFFECTS — Naloxone can precipitate acute withdrawal symptoms in opioid-dependent patients; the risk may be greater with higher doses. Acute opioid withdrawal is associated with anxiety, piloerection, yawning, sneezing, rhinorrhea, nausea, vomiting, diarrhea, and abdominal or muscle cramps. Naloxone-induced noncardiogenic pulmonary edema has been reported rarely and appears to be more common when higher doses are used.6
In the two pharmacokinetic studies in healthy adults, abdominal pain, asthenia, dizziness, headache, nasal discomfort, and presyncope were reported in a few of the subjects who received intranasal naloxone 8 mg. Signs of nasal inflammation and nasal congestion were also observed.
PREGNANCY — No embryotoxic or teratogenic effects occurred in pregnant mice and rats treated with large doses of naloxone. Naloxone crosses the placenta; it may induce preterm labor and cause opioid withdrawal symptoms in the fetus of an opioid-dependent mother.
DOSAGE AND ADMINISTRATION — Kloxxado is supplied in cartons containing two 8 mg/0.1 mL single-use nasal spray devices. The recommended dosage for adults and children is 8 mg administered as a single spray into one nostril. Additional doses can be given every 2 to 3 minutes in alternating nostrils using a new device for each dose. No assembly or priming of the device is required.
AVAILABILITY — Every state in the US now has a naloxone access law that allows an individual to obtain naloxone without a personal prescription. These laws may also grant civil and criminal immunity to laypersons who administer naloxone and to healthcare professionals who prescribe or dispense the drug to laypersons.
- Naloxone (Narcan) nasal spray for opioid overdose. Med Lett Drugs Ther 2016; 58:1.
- CDC. Health Alert Network (HAN). Increase in fatal drug overdoses across the United States driven by synthetic opioids before and during the COVID-19 pandemic. December 17, 2020. Available at: https://bit.ly/3AU8nCZ. Accessed August 26, 2021.
- D Khoury et al. Increases in naloxone administrations by emergency medical services providers during the COVID-19 pandemic: retrospective time series study. JMIR Public Health Surveill 2021; 7:e29298.
- HHS. Naloxone: the opioid reversal drug that saves lives. Available at: https://bit.ly/3k68NiV. Accessed August 26, 2021.
- Drugs for opioid use disorder. Med Lett Drugs Ther 2017; 59:89.
- S Elkattawy et al. Naloxone induced pulmonary edema. J Community Hosp Intern Med Perspect 2021; 11:139.