ISSUE1298
Inhaled drugs for asthma are available in the US mainly in pressurized metered-dose inhalers (MDIs), which require a propellant, and dry powder inhalers, which do not. The chlorofluorocarbon (CFC) propellants in MDIs are being replaced by hydrofluoroalkane (HFA) propellants for environmental reasons: CFCs contribute to the depletion of the ozone layer. December 31, 2008 will be the last day that albuterol MDIs using CFC propellants can be sold in the US. The FDA is expected to announce a termination date for other CFC-containing products in the near future.
Three HFA albuterol inhalers and one HFA levalbuterol inhaler have been approved by the FDA. None is available generically. HFA inhalers require priming — firing 4 puffs into the air (3 with ProAir) — the first time they are used, and after 2 weeks of non-use (3 days with Xopenex HFA).
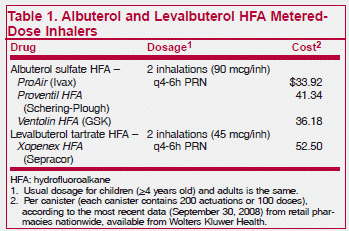
In general, HFA sprays taste different, are less forceful, and are warmer and mistier than CFC sprays. Some patients may have to be reassured that they are getting enough of their medication, but actually the smaller particles of the HFA sprays may reach the lungs more readily than CFC sprays.
