ISSUE1616
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- F. Peter Swanson, M.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Explain the current approach to the treatment of community-acquired pneumonia.
- Discuss the pharmacologic options available for treatment of community-acquired pneumonia and compare them based on their efficacy, dosage and administration, and potential adverse effects.
- Determine the most appropriate therapy given the clinical presentation of an individual patient with community-acquired pneumonia.
Treatment of community-acquired pneumonia (CAP) is usually empiric, with selected antibiotic regimens directed against some of the most common causative pathogens. Recommended empiric regimens are listed in Table 2; recommended antibiotic dosages for treatment of CAP are listed in Tables 3 and 4. Joint guidelines for treatment of CAP by the American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) were updated in 2019.1
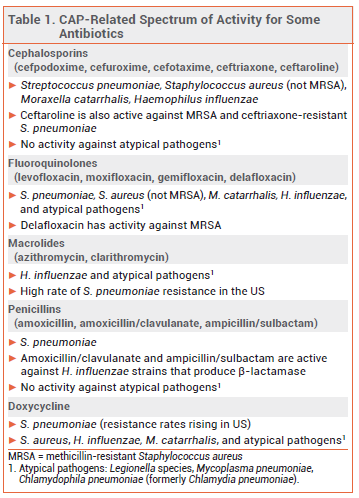
BACTERIAL PATHOGENS — The organisms responsible for CAP are often not identified, but Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, and "atypical" organisms (Mycoplasma pneumoniae, Legionella species, and Chlamydophila pneumoniae) have traditionally been the most common causative bacterial pathogens. In the US, pneumococcal vaccination has substantially reduced the rates of respiratory and other infections caused by S. pneumoniae.2
Methicillin-resistant S. aureus (MRSA) and resistant gram-negative organisms, such as Pseudomonas aeruginosa, can cause CAP. Patients with previous respiratory isolation of one of these pathogens and/or recent hospitalization and parenteral antibiotic treatment are at increased risk.1
INFLUENZA AND COVID-19 — Influenza and SARSCoV-2 can also cause CAP. While these viruses are circulating in the community, patients with symptoms of acute respiratory illness should be tested for both viruses and receive appropriate treatment based on test results, risk of complications, and severity of illness.3-5 If secondary bacterial pneumonia is suspected, empiric antibiotic therapy should be started.6
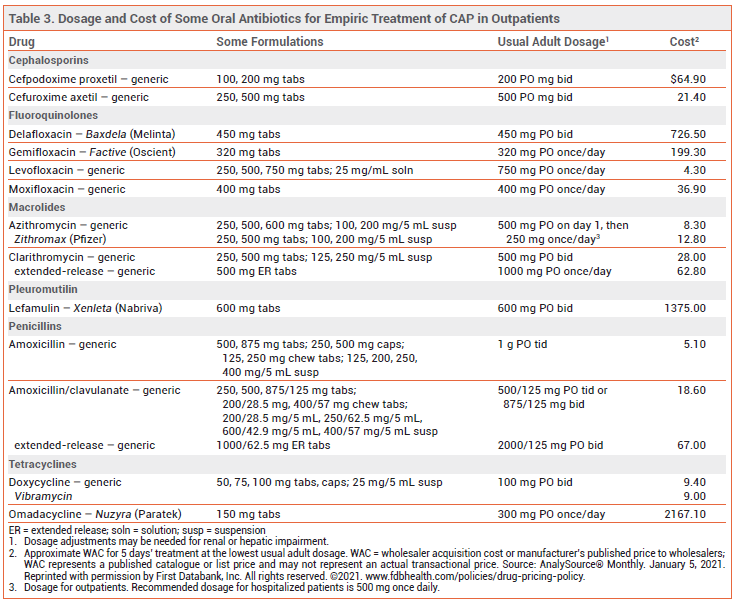
TREATMENT OF OUTPATIENTS — For outpatient treatment of CAP in otherwise healthy adults without comorbidities (chronic heart, lung, liver or renal disease, diabetes, alcoholism, malignancy, or asplenia), current guidelines recommend oral treatment with high-dose amoxicillin (1 g tid) or doxycycline (see Table 2). For many years, monotherapy with an oral macrolide such as azithromycin was a regimen of choice for treatment of CAP, but the rate of macrolide-resistant S. pneumoniae is now >40% in many areas in the US; current guidelines recommend that oral macrolide monotherapy be considered only in areas where pneumococcal resistance rates to macrolides are <25%.1 High-dose amoxicillin monotherapy provides coverage against S. pneumoniae, but it has no activity against atypical pathogens; some experts would add a macrolide or doxycycline if infection with an atypical pathogen is suspected.
For outpatient treatment of CAP in adults with comorbidities (chronic heart, lung, liver or renal disease, diabetes, alcoholism, malignancy, or asplenia), oral treatment with a combination of a beta-lactam (amoxicillin/clavulanate, cefpodoxime, or cefuroxime) and a macrolide (azithromycin, clarithromycin) or doxycycline is recommended. Monotherapy with an oral respiratory fluoroquinolone (gemifloxacin, levofloxacin, or moxifloxacin) is another option. Patients with comorbidities, who are more likely to have had healthcare system encounters and previous antibiotic exposure, are at increased risk of infection with resistant pathogens including H. influenzae, M. catarrhalis, S. aureus (including MRSA), and gram-negative bacilli. Patients recently treated with an antibiotic from one of the classes included in the above regimens should receive an antibiotic from a different class.1
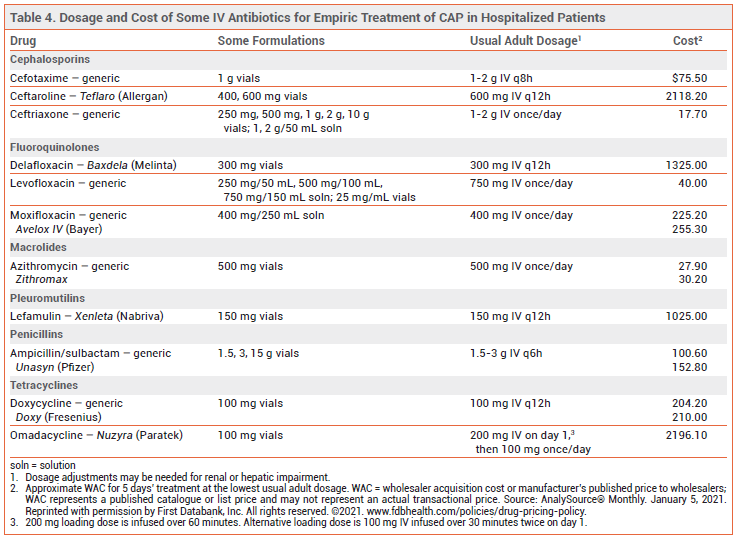
TREATMENT OF HOSPITALIZED PATIENTS — In patients who are hospitalized for nonsevere CAP, empiric treatment with a combination of an IV beta-lactam (ampicillin/sulbactam, ceftriaxone, cefotaxime, or ceftaroline) and an IV or oral macrolide (azithromycin, clarithromycin) or monotherapy with an IV or oral respiratory fluoroquinolone (levofloxacin, moxifloxacin) is recommended. In patients who cannot take a macrolide or a fluoroquinolone, an IV beta-lactam plus IV or oral doxycycline is an alternative.
In patients who are hospitalized for severe CAP and do not have risk factors for infection with P. aeruginosa (or other resistant gram-negative pathogens) or MRSA, empiric treatment with an IV beta-lactam (ampicillin/sulbactam, cefotaxime, ceftriaxone, or ceftaroline) plus either an IV macrolide (azithromycin) or an IV respiratory fluoroquinolone (levofloxacin, moxifloxacin) is recommended.
MRSA AND OTHER RESISTANT PATHOGENS — Hospitalized patients with CAP who have risk factors for infection with MRSA or resistant gram-negative organisms such as P. aeruginosa may be treated empirically for such pathogens in addition to standard empiric treatment. For empiric coverage of MRSA, IV vancomycin or linezolid can be added to a recommended empiric regimen (see Table 2). For empiric coverage of P. aeruginosa and other gram-negative pathogens, a broad-spectrum IV beta-lactam that is also effective against S. pneumoniae, such as piperacillin/tazobactam or cefepime, or a carbapenem, such as imipenem, can be used. The regimen should also include an agent with activity against Legionella species and other atypical organisms, such as a respiratory fluoroquinolone. Cultures and/or nasal PCR should be performed to confirm the need for continued treatment.
ADVERSE EFFECTS — Beta-lactam antibiotics (penicillins and cephalosporins) can cause rash, diarrhea, nausea, vomiting, allergic reactions, hemolytic anemia, neutropenia, cholestatic hepatitis, serum sickness, and seizures.
Doxycycline can cause GI disturbances and photosensitivity. Use in children <8 years old can inhibit bone growth and cause permanent discoloration of teeth and enamel hypoplasia.
Azithromycin and clarithromycin can cause GI disturbances, headache, dizziness, vaginitis, and QT-interval prolongation. Clarithromycin can also cause dysgeusia and hepatic enzyme elevations. The FDA has warned that use of clarithromycin may increase the risk of cardiovascular morbidity and mortality in patients with heart disease.7
Fluoroquinolones can cause GI disturbances, tremors, rash, oral and vaginal Candida infections, eosinophilia, neutropenia, leukopenia, increased aminotransferase and serum creatinine levels, insomnia, photosensitivity reactions, and peripheral neuropathy. They have also been associated with hyperglycemia and severe hypoglycemia, especially in older adults and in patients with diabetes. Central nervous system effects including seizures, delirium, agitation, nervousness, and disturbances in attention, memory, and orientation have occurred. Other serious adverse effects include tendinitis, tendon rupture, aortic aneurysm, exacerbation of myasthenia gravis, Clostridioides difficile-associated diarrhea, and (except for delafloxacin) QT-interval prolongation and torsades de pointes.8
DRUG INTERACTIONS — Coadministration of antacids or products containing calcium, magnesium, or iron can decrease absorption of doxycycline and fluoroquinolones. Administration should be separated by several hours.
Concurrent use of azithromycin, clarithromycin, or fluoroquinolones with other QT-interval-prolonging drugs can result in additive effects.9
Use of fluoroquinolones with antihyperglycemic drugs may increase the risk of hypoglycemia. Concurrent use of fluoroquinolones and nonsteroidal anti-inflammatory drugs (NSAIDs) may lower the seizure threshold.
PREGNANCY — Amoxicillin or amoxicillin/clavulanate plus azithromycin can be used for outpatient treatment of pregnant women with CAP. Pregnant wo men who are hospitalized for CAP can be treated with an IV beta-lactam plus azithromycin.
Doxycycline, clarithromycin, and fluoroquinolones should be avoided, if possible, in pregnant women. Doxycycline can cause fetal tooth discoloration and enamel hypoplasia and can inhibit bone growth. Clarithromycin is teratogenic in animals. Fluoroquinolones have caused arthropathy in animal studies, but observational data in pregnant women suggest that teratogenic effects are unlikely to occur at therapeutic doses.
NEWER ANTIBIOTICS — Data supporting the efficacy of newer FDA-approved antibiotics for treatment of CAP, including the fluoroquinolone delafloxacin (Baxdela), the tetracycline omadacycline (Nuzyra),10 and the pleuromutilin lefamulin (Xenleta),11 are limited. Until more data become available, empiric regimens with a longer record of efficacy and safety are preferred.
DURATION OF ANTIMICROBIAL THERAPY — Antibiotic treatment should be continued until clinical stability is achieved (usually within 48-72 hours) and for at least 5 days. Short courses of treatment (5-7 days) appear to be similar in efficacy to longer courses (8-10 days).12 When switching from IV to oral therapy, the same drug or a drug from the same class should be used.
ADJUNCTIVE CORTICOSTEROIDS — No data are available supporting the use of adjunctive corticosteroids for treatment of mild to moderate CAP. Data on whether they improve clinical outcomes in patients with severe CAP are mixed; until more evidence becomes available, they probably should not be used routinely, except in patients with CAP and refractory septic shock.13
- JP Metlay et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45.
- Adult immunization. Med Lett Drugs Ther 2018; 60:73.
- Antiviral drugs for influenza for 2020-2021. Med Lett Drugs Ther 2020; 62:169.
- National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://covid19treatmentguidelines.nih.gov/. Accessed January 7, 2021.
- JP Metlay and GW Waterer. Treatment of community-acquired pneumonia during the coronavirus disease 2019 (COVID-19) pandemic. Ann Intern Med 2020; 173:304.
- Treatments considered for COVID-19. Med Lett Drugs Ther 2020. Available at: https://secure.medicalletter.org/downloads/1595e_table.pdf. Accessed January 7, 2021.
- FDA Drug Safety Communication: FDA review finds additional data supports the potential for increased long-term risks with antibiotic clarithromycin (Biaxin) in patients with heart disease. February 22, 2018. Available at: https://bit.ly/3omrXlH. Accessed January 7, 2021.
- In brief: More fluoroquinolone warnings. Med Lett Drugs Ther 2018; 60:136.
- RL Woosley et al. QT drugs list. Available at: www.crediblemeds.org. Accessed January 7, 2021.
- Omadacycline (Nuzyra) – a new tetracycline antibiotic. Med Lett Drugs Ther 2019; 61:74.
- Lefamulin (Xenleta) for community-acquired bacterial pneumonia. Med Lett Drugs Ther 2019; 61:145.
- GS Tansarli and E Mylonakis. Systematic review and meta-analysis of the efficacy of short-course antibiotic treatments for community-acquired pneumonia in adults. Antimicrob Agents Chemother 2018; 62:e00635-18.
- Corticosteroids in community-acquired pneumonia. Med Lett Drugs Ther 2020; 62:7.